Limiting chiral chromatography stationary phase material and preparation method thereof
A chiral chromatography and stationary phase technology, which is used in the synthesis of new chromatographic separation materials to reduce losses, improve efficiency and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Synthesis of methyl butynyl methacrylate
[0024] Methylbutynol (21.0 mL, 217 mmol), triethylamine (29.8 mL, 207 mmol) were dissolved in dry dichloromethane (120 mL) and stirred, and the solution was placed in an ice-water bath and lowered to 0°C, Methacryloyl chloride (20.0 mL, 207 mmol) was added dropwise, and after reacting for 0.5 hours, the mixture was stirred at room temperature for 20 hours. The solution was washed twice with 1% HCl (weight percent concentration), saturated sodium bicarbonate solution and water respectively, the organic phase was dried with anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain methyl butynyl methacrylate (MBMA for short) , the yield is 74%.
[0025] 2. Grafting ATRP initiator on the surface of silicon spheres
[0026] Pretreatment of matrix silica gel: porous silica gel microspheres (particle size 10 μm, pore size ) 6.0 g was added to the hydrochloric acid / water (1:1, V / V) solution, stirred ...
Embodiment 2
[0029] 1. Initiate ATRP on the surface of silica gel and graft poly(methyl butynyl methacrylate) (the product is abbreviated as pMBMA-sil)
[0030] Weigh 2.1 g of Br-sil prepared in Example 1 into a round-bottomed three-necked flask, add 0.83 ml (5.49 mmol) of methylbutynyl methacrylate and 30 ml of cyclohexanone, pass N 2 After 10 minutes, add 2,2'-bipyridine 0.0343 g (0.22 mmol) and CuBr 0.0158 g (0.11 mmol), N 2 The reaction was carried out at room temperature for 4 hours under protection. After the reaction is completed, the reaction solution is poured into ethanol to stop the polymerization reaction, and the copper ions adsorbed on the surface of the silicon spheres are washed away with a saturated aqueous solution of disodium EDTA, and then the unreacted substances are washed away with ethanol and acetone, and vacuum dried. , to obtain pMBMA-sil, and the number of repeating units n of grafted pMBMA was calculated to be 4.0 according to the increase in carbon content fro...
Embodiment 3
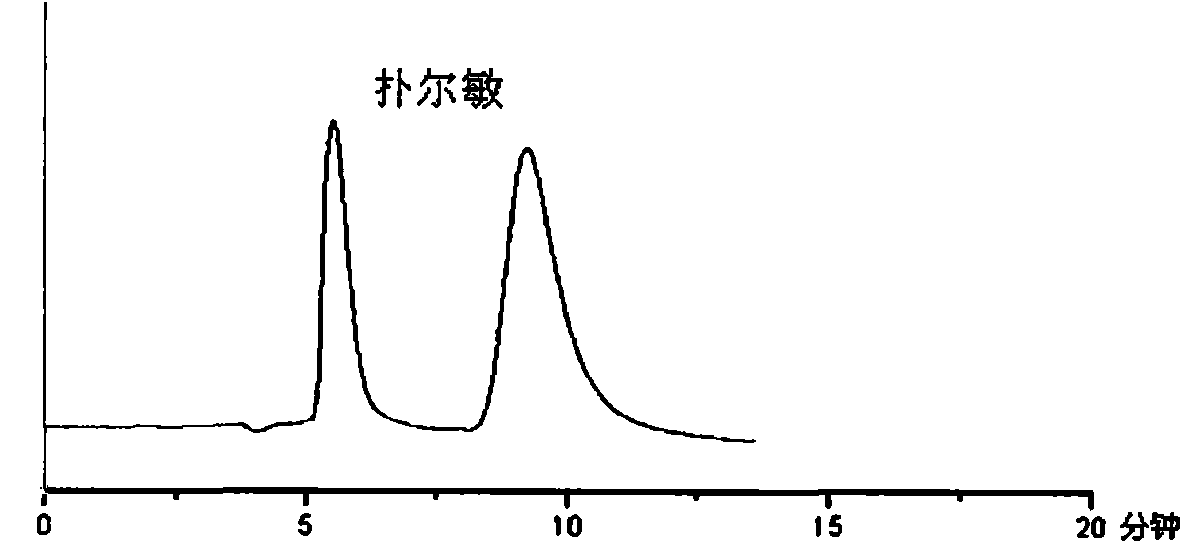
[0039] The chiral separation material (CD-sil) synthesized in Example 2 was loaded into a stainless steel chromatographic column of 150mm × 4.6mm i.d. by homogenization method, and the HPLC method was used to investigate the chiral drugs chlorthalidone and mandelic acid of this material. , chlorpheniramine and benzoin separation ability. The chromatographic conditions and separation results are shown in Table 1, and the chromatographic separation diagram is shown in Table 1. Figure 1-4 . The chiral selectivity alpha values for the four drugs were >1.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com