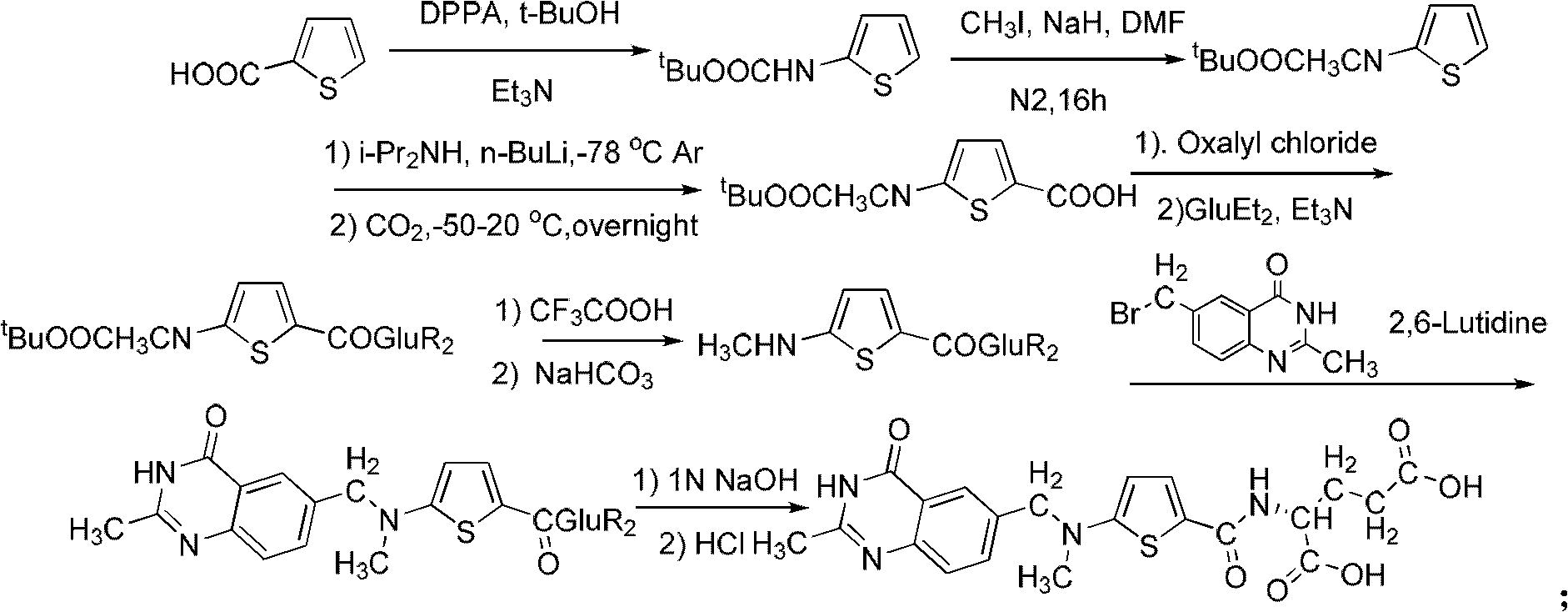
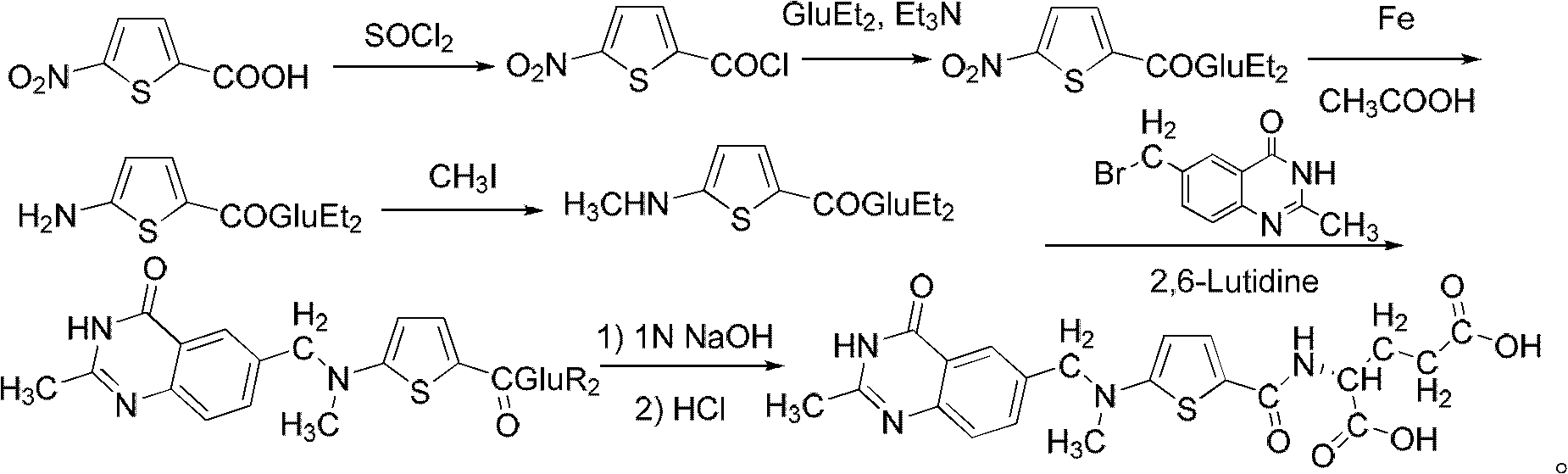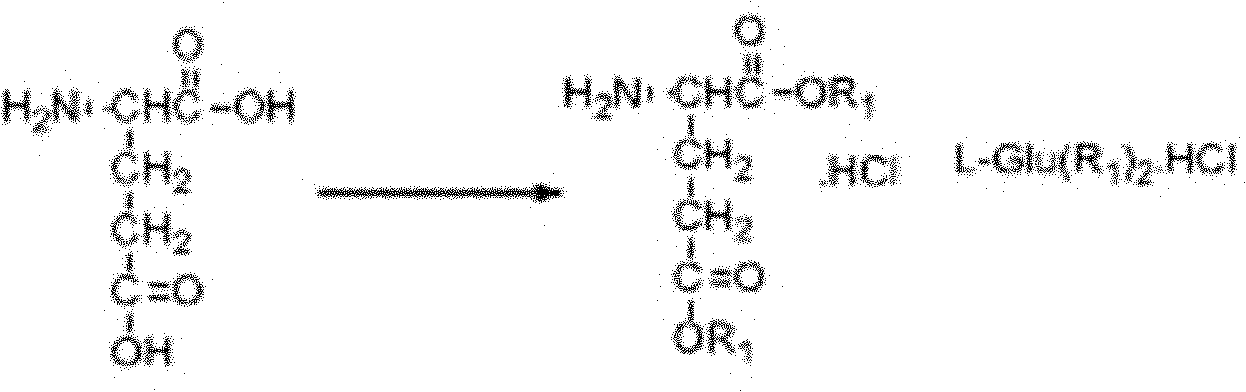New synthesis technology of anti-cancer drug Raltitrexed
A technology of raltitrexed and anticancer drugs, which is applied in the field of chemical synthesis of drugs, can solve the problems of difficult separation of monomethylated products, difficulty in controlling methylated products, and harsh reaction conditions, so as to achieve easy control of the reaction, Low cost and the effect of increasing the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0052] 1. Synthesis of L-glutamic acid diethyl ester hydrochloride
[0053] Add 60 mL of freshly distilled anhydrous ethanol to a 100 mL three-neck flask connected with a spherical condenser (connected to the drying pipe and tail gas receiving device) and a constant pressure dropping funnel, and dissolve 26 mL of freshly distilled SOCl in an ice-salt bath at -10 to -15°C 2 (366mmol) was dropped into the system through a constant pressure dropping funnel for 0.5h, then warmed up to room temperature, and stirred for 3h. Add 8.8 g (56.2 mmol) of L-glutamic acid to the system, and react overnight at room temperature. Raise the temperature to 65°C, reflux for about 1.5 hours, and the insoluble white suspension turns into a light brown transparent liquid. The reaction is monitored by TLC, and the reaction is complete after 7 hours. Stop the reaction, quench, and distill off excess SOCl 2 and ethanol, solidified in an ice-water bath, and dried in an oven at 44°C for 9 hours to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com