Traditional Chinese medicine composition for treating iron deficiency anemia and preparation method thereof
A technology for iron-deficiency anemia and a composition, applied in the field of pharmacy, can solve problems such as iron malabsorption, and achieve the effects of less dosage, significant curative effect, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, granules of the present invention
[0042] Prescription: soap alum 4.5g, astragalus 270g, hawthorn 270g, new donkey-hide gelatin 90g, jujube 90g
[0043] Preparation method: decoct astragalus, hawthorn, and jujube with water twice, 2 hours each time, add water 10 times and 8 times respectively, combine the decoctions, concentrate to a clear paste with a relative density of 1.08 (measured at 80°C), add Equal amount (calculated by volume) of 95% ethanol, let it stand for 48 hours or centrifuge, concentrate the supernatant or centrifugate to a clear paste with a relative density of 1.29-1.31 (measured at 80°C), and filter. Crush soap alum and new donkey-hide gelatin, mix the fine powder with the above-mentioned clear paste, sugar powder, and dextrin, and make granules to obtain. (1000g granules)
Embodiment 2
[0044] Embodiment 2, drug effect test of the present invention
[0045] 1 Materials and methods
[0046] 1.1 Sample
[0047] Granules of the present invention: Example 1 of the present invention, the specification is 10g / bag; ferrous sulfate sheet, containing ferrous sulfate is 0.3g / sheet, provided by Jinan Yongning Pharmaceutical Co., Ltd., batch number 20051211.
[0048] 1.2 Experimental animals
[0049] A total of 70 SPF-grade female weaned Wistar rats, weighing 50-60 g, were selected from the Experimental Animal Center of Shandong Medical University. The breeding environment is a barrier-level animal room, and the license number for the use of experimental animals is: SYXK-(Lu) 2006-0025. Low-iron feed was provided by the Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention.
[0050] 1.3 Dose selection
[0051] The granules of the present invention are provided with low and high dosage groups, which are respectively 0.5 and 1.0 g / k...
Embodiment 3
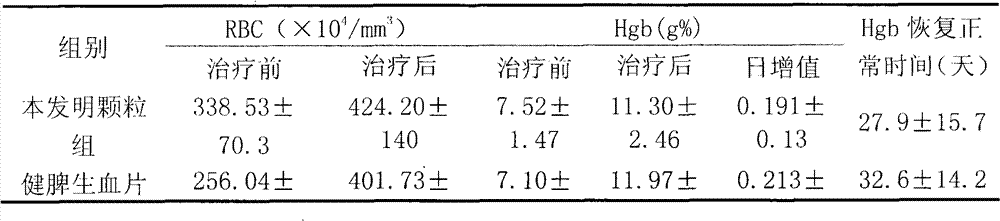
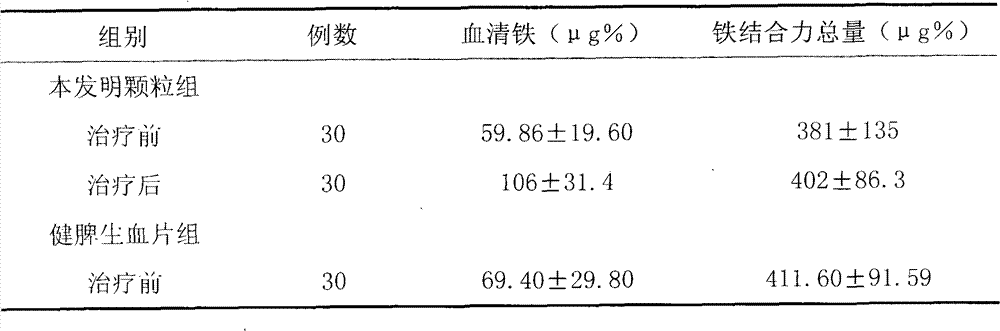
[0085] Embodiment 3, clinical observation result report is as follows:
[0086] clinical information
[0087] A total of 224 cases of iron deficiency anemia were treated. Among them, there were 32 males and 192 females; the age ranged from 10 months to 79 years, with an average age of 36 years; the time since the onset of symptoms came to the hospital for 1 month to 22 years, and the average disease duration was 5.6 years.
[0088] 224 cases were divided into three groups for observation: 101 cases in the granule group of the present invention, 93 cases in the Jianpi Shengxue Tablet group, and 30 cases in the ferrous sulfate group. Before each case takes the medicine, fill in the observation form of pre-selected design. The content of the form mainly includes the detection values of hemoglobin, red blood cell count and other clinical symptoms related to anemia.
[0089] Treatments and Outcomes
[0090] 1. Treatment
[0091] 1. Drug composition: Granules of the present in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com