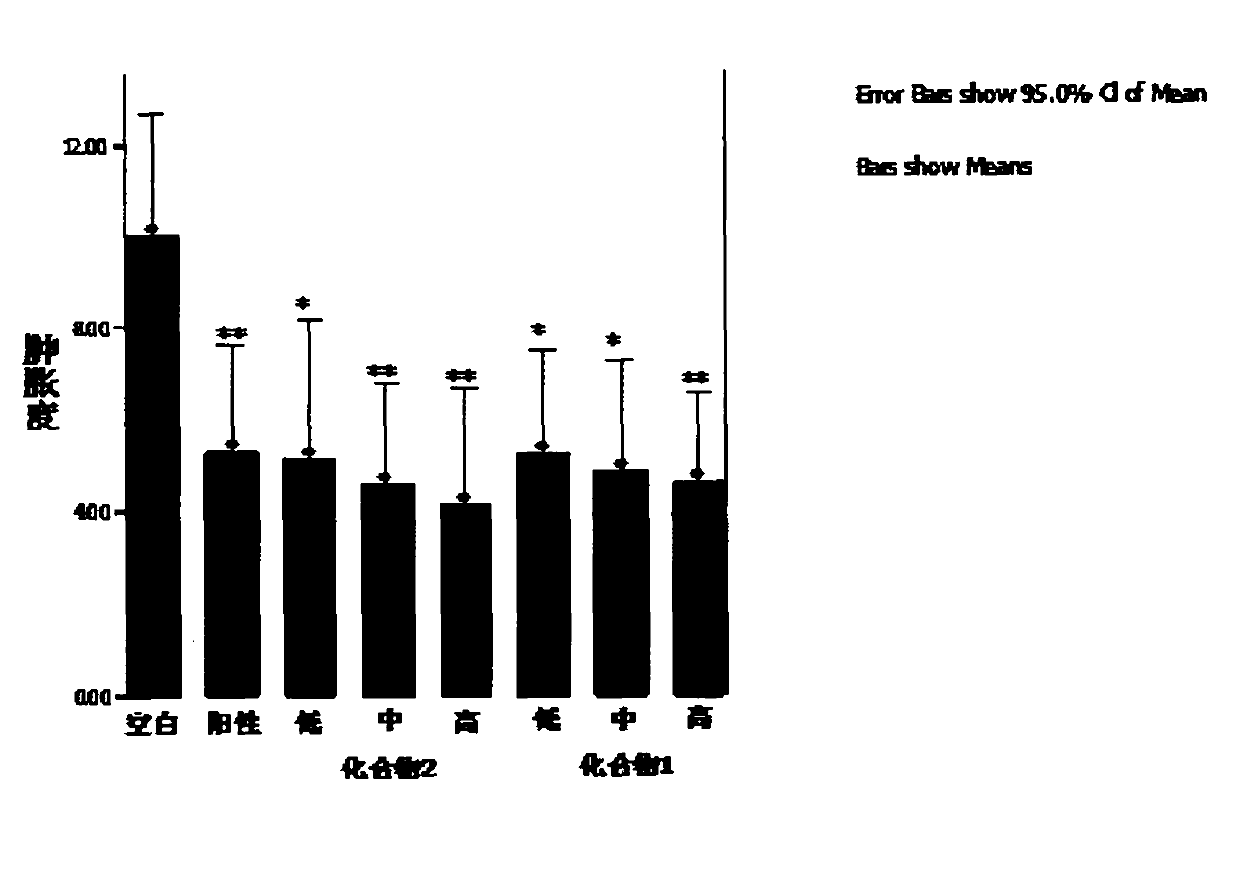
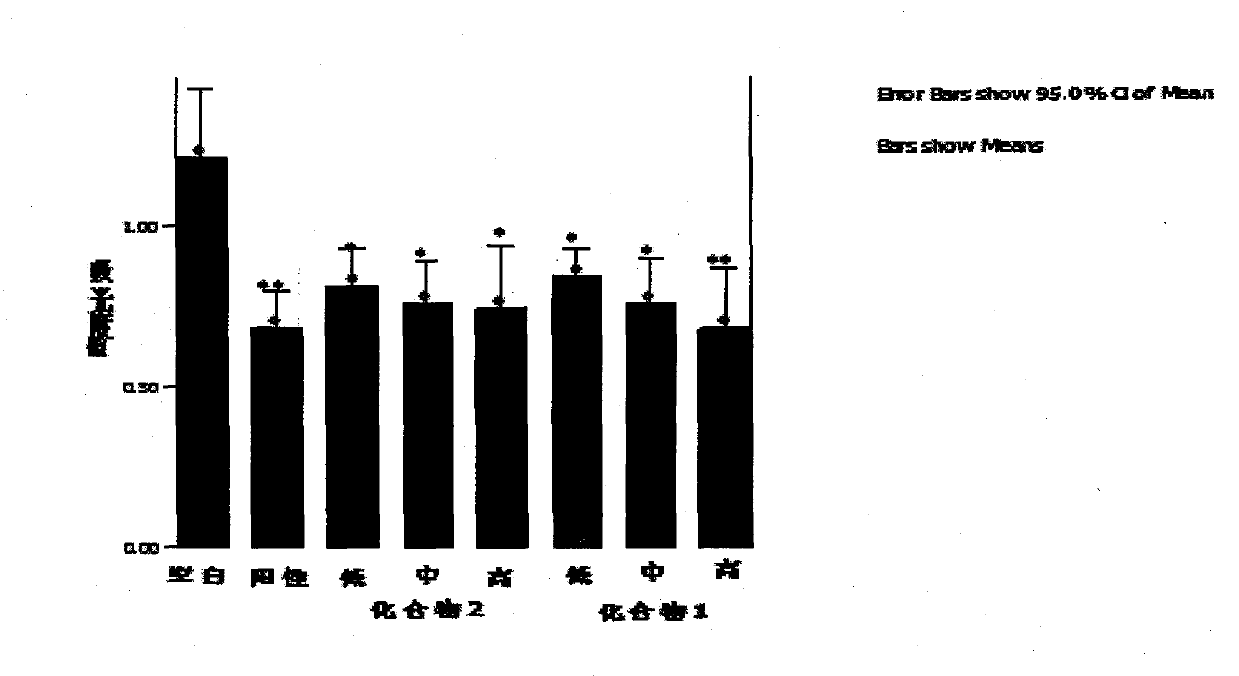
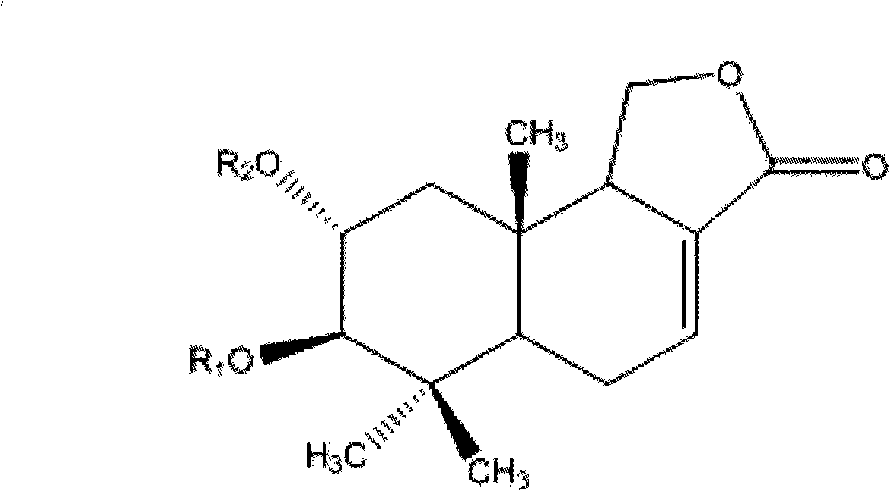
2, 3-dihydroxy cinnamomum lactone derivative and application thereof
A technology of dihydroxy and derivatives, applied in 2 fields, can solve the problems that the pharmacological effects have not been reported yet, and achieve good anti-inflammatory activity, good stability, and clear activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A method for preparing 2,3-dihydroxycinnamon lactone derivatives, the specific steps are as follows: the dried whole plant of Polygonum jucundum Meisn. is pulverized, extracted by heating and refluxing with 90% ethanol, recovering the organic solvent under reduced pressure, and sequentially using petroleum Ether, ethyl acetate extraction, ethyl acetate part extract through silica gel column chromatography, petroleum ether-ethyl acetate gradient elution (petroleum ether: ethyl acetate 15:1 ~ 9:1 ~ 5:1 ~ 3:1 ~2:1~1:1~methanol unloading column), wherein petroleum ether-ethyl acetate (2:1) was eluted to obtain compound 2; wherein the methanol eluted part was subjected to silica gel column chromatography, chloroform-methanol gradient elution, Chloroform:methanol (20:1) was eluted to obtain compound 1, and the above two substances were recrystallized from ethanol-water to obtain pure compound.
[0017] Compound 1 white needle crystal (methanol). mp134-136°C. GF under 254nm ...
Embodiment 2
[0024] Take 0.5g of compound 1, add 10ml of 20ml of absolute ethanol, stir, add 5ml of concentrated sulfuric acid, stir for 72 hours, add 20ml of 95% ethanol, adjust the pH to 7 with 50% NaoH, adjust the volume fraction of ethanol to 85%, and let it stand for reaction 36h, filtered, separated by D101 resin (50%-70% ethanol gradient elution) and ODS column chromatography to obtain compounds 3 and 4.
[0025] Take 1g of compound 2, add 25ml of 50ml of absolute ethanol, stir, add 10ml of concentrated sulfuric acid, stir for 72 hours, add 40ml of 95% ethanol, adjust the pH to 7 with 50% NaoH, adjust the volume fraction of ethanol to 85%, and let it stand for overnight reaction , filtered, and separated by D101 resin (70% ethanol gradient elution) and ODS column chromatography to obtain compound 5.
[0026] Compound 3 white powder. mp165-167°C. UV(methanol)λ max : 230.4nm. [α] 25 D -13.6 (c=0.1, MeOH). IR (KBr): 3426, 1750, 1646, 1465, 1035cm -1 , 1 H-NMR (CD 3OD, 300MHz)...
Embodiment 3
[0033] Take 0.2 g of compound 1, add 3 ml of dichloromethane, cool in an ice bath, add 0.3 ml of trifluoroacetic anhydride, stir for 2 h, and separate by silica gel column chromatography (petroleum ether-ethyl acetate 1:1 elution) to obtain compound 6 ( Formula I, R 1 = R 2 = CF 3 CO).
[0034] Take 0.2g of compound formula 2, add 5ml of dichloromethane, cool in an ice bath, add 0.5ml of trifluoroacetic anhydride, stir for 4 hours, and separate by silica gel column chromatography (petroleum ether-ethyl acetate 1:1 elution) to obtain compound 7 (formula I, R1=angeloyl, R2=CF 3 CO).
[0035] Compound 6 is white powder. mp130-132°C. UV(methanol)λ max : 222.4nm. IR (KBr):, 2913, 1757, 1692, 1178cm -1 . 1 H-NMR (CD 3 Cl, 300MHz) δ1.89 (1H, dd, J=12.6, 4.3Hz, H-1β), 1.28 (1H, t, J=12.6, 12.1Hz, H-1α), 4.05 (1H, ddd, J= 12.1, 4.3, 9.5Hz (H-2), 2.95 (1H, d, J=9.5Hz, H-3), 1.53 (1H, dd, J=11.5, 5.5Hz, H-5), 2.32 (1H, m , H-6β), 2.49 (1H, m, H-6α), 6.88 (1H, m, H-7), 2.96 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com