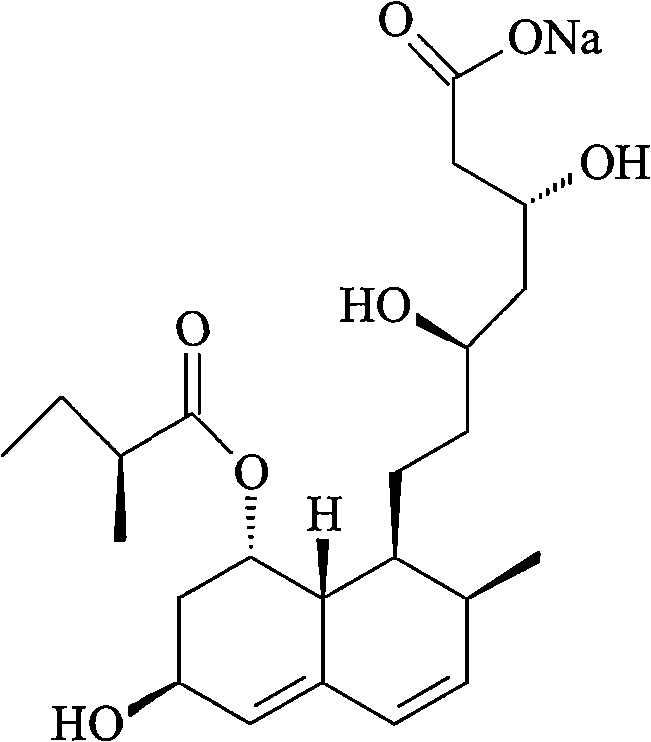
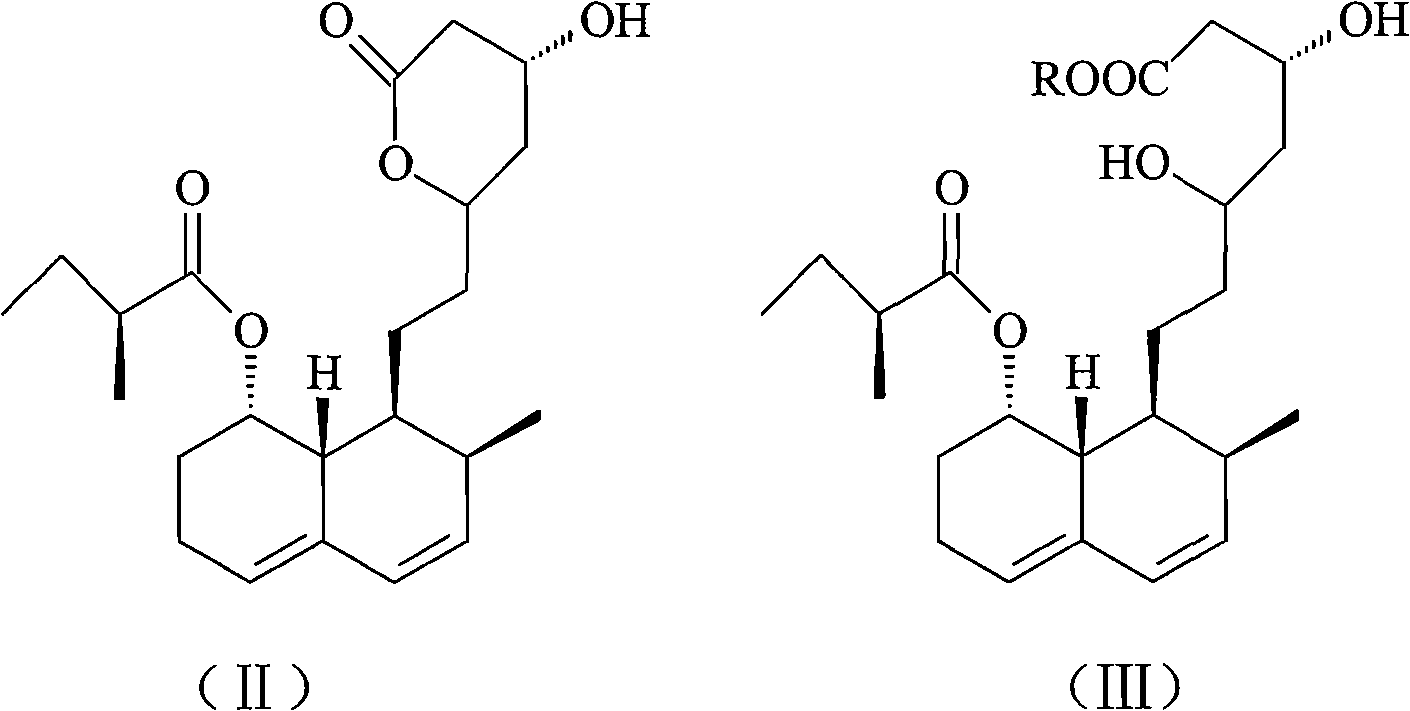
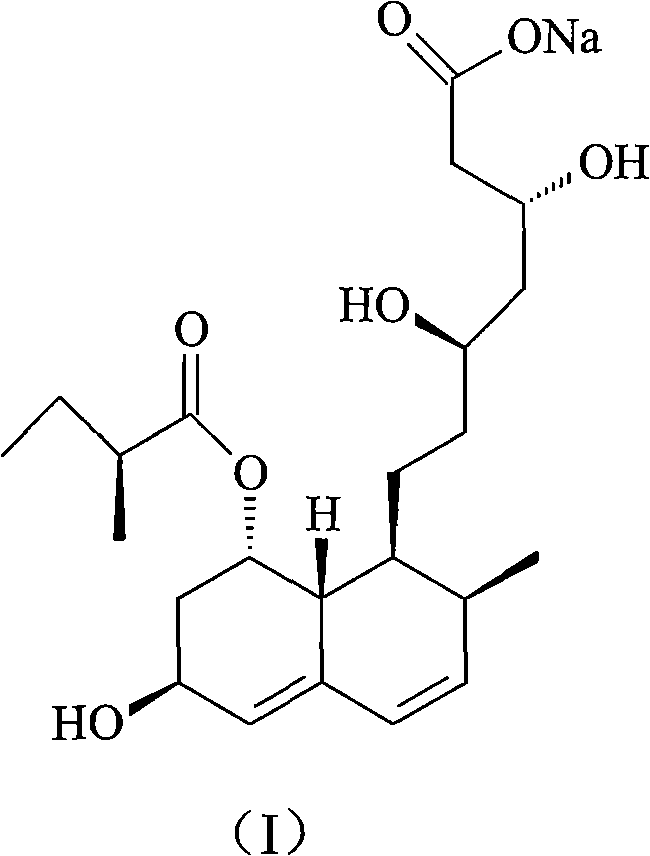
Pravastatin sodium compound and novel preparation method thereof
A technology of pravastatin sodium and pravastatin is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, and can solve the problems of low purity of pravastatin sodium compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The refining of embodiment 1 pravastatin sodium
[0056] 10 g of crude pravastatin sodium (purity 98.4%) was dissolved in 200 ml of water, added into a fixed bed filled with D001 type strongly acidic styrene-based cation exchange resin, and continued to exchange for 1.5 hours.
[0057] Elution was carried out with 1% aqueous sodium bicarbonate solution at an elution rate of 1.0 ml / min, the eluate was collected, and the eluate was filtered to obtain the elution mother liquor.
[0058] The eluting mother liquor is passed through the chromatographic column, and the packing agent as the stationary phase is fine-pore silica gel with a particle diameter of 80-200 μm and a pore diameter of 40-80A. The length of the column is 20 cm, and the diameter is 3 cm. : 1) Carry out column chromatography, flow rate is 2.5ml / min, column temperature keeps 30 ℃, start timing, take a sample, track and monitor, carry out segmental collection, collect liquid, wash with water, dry over anhydr...
Embodiment 2
[0059] The refining of embodiment 2 pravastatin sodium
[0060] 10 g of pravastatin sodium crude product (purity 98.2%) was dissolved in 200 ml of water, added to a fixed bed filled with D001 type strongly acidic styrene-based cation exchange resin, and continued to exchange for 1.5 hours.
[0061] Elute with 0.5% aqueous sodium bicarbonate solution at an elution rate of 0.8 ml / min, collect the eluate, and filter the eluate to obtain the eluted mother liquor.
[0062] The eluting mother liquor is passed through the chromatographic column, and the packing agent as the stationary phase is fine-pore silica gel with a particle diameter of 80-200 μm and a pore diameter of 40-80A. The length of the column is 20 cm, and the diameter is 3 cm. 0.85:1) for column chromatography, the flow rate is 2.0ml / min, the column temperature is kept at 30°C, start timing, sampling, follow-up monitoring, segmental collection, collect the liquid, wash with water, dry over anhydrous sodium sulfate, a...
Embodiment 3
[0063] The refining of embodiment 3 pravastatin sodium
[0064] 20g of crude pravastatin sodium (purity 97.9%) was dissolved in 200ml of water, added into a fixed bed filled with D001 type strongly acidic styrene-based cation exchange resin, and continued to exchange for 1.5 hours.
[0065] Elute with 0.2% sodium carbonate aqueous solution at an elution rate of 1.2 ml / min, collect the eluate, filter the eluate, and obtain the eluted mother liquor.
[0066] The eluting mother liquor is passed through the chromatographic column, and the filler as the stationary phase is fine-pore silica gel with a particle diameter of 80-200 μm and a pore diameter of 40-80A. The length of the column is 20 cm, and the diameter is 3 cm. : 1) Carry out column chromatography, flow velocity is 0.5ml / min, column temperature keeps 30 ℃, start timing, take a sample, track and monitor, carry out segmental collection, collect liquid, wash with water, dry over anhydrous sodium sulfate, concentrate under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com