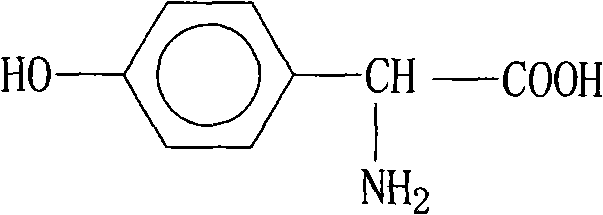
Method for synthesizing p-hydroxyphenylglycine
A technology of p-hydroxyphenylglycine and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems such as color and purity that cannot meet the requirements of splitting, complicated process operation, and increased cost, etc. Achieve the effect of low cost, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 247 grams of 30% glyoxylic acid is converted to 74 grams of glyoxylic acid, i.e. 1 mole; 85 grams of phenol is taken, i.e. 0.9 moles, 0.85 g of toluenesulfonic acid, and the above-mentioned phenol, glyoxylic acid, toluenesulfonic acid One-time input at 35°C, the reaction time is 20 hours; add 0.094 g of sodium bisulfite to change the color, adjust the pH to 3 with sodium hydroxide solution, control the reaction temperature at 25°C, and crystallize for 1 hour at 0°C ; The crystals were washed with a large amount of water, and then washed with the organic solvent methanol to obtain p-hydroxyphenylglycine.
[0032] It has the characteristics of simple process, low cost, high yield up to 88%, uniform color and purity, and can meet the requirements of separation.
Embodiment 2
[0034] Get 740 grams of 10% glyoxylic acid, equivalent to 74 grams of glyoxylic acid, i.e. 1 mole; get 186 grams of phenol, i.e. 2 moles, 0.0186 grams of benzenesulfonic acid, the above-mentioned phenol, glyoxylic acid, benzenesulfonic acid One-time input at 75°C, the reaction time is 5 hours; add 0.186 g of sodium sulfite to change the color, adjust the pH to 6 with ammonia solution, control the reaction temperature at 25°C, and the crystallization time is 10 hours at 40°C; Wash with water, and then rinse with organic solvent ethanol to obtain p-hydroxyphenylglycine.
[0035] It has the characteristics of simple process, low cost, high yield, uniform color and purity, and can meet the requirements of separation.
Embodiment 3
[0037] 247 grams of 30% glyoxylic acid is converted into 74 grams of glyoxylic acid, i.e. 1 mol; Add sulfonic acid at 50°C once, and the reaction time is 10 hours; add 0.186 g of hydroxylamine hydrochloride to change the color, adjust the pH to 6 with lithium hydroxide solution, control the reaction temperature at 50°C, and crystallize for 5 hours at 20°C ; The crystals were washed with a large amount of water, and then washed with the organic solvent acetone to obtain p-hydroxyphenylglycine.
[0038] It has the characteristics of simple process, low cost, high yield, uniform color and purity, and can meet the requirements of separation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com