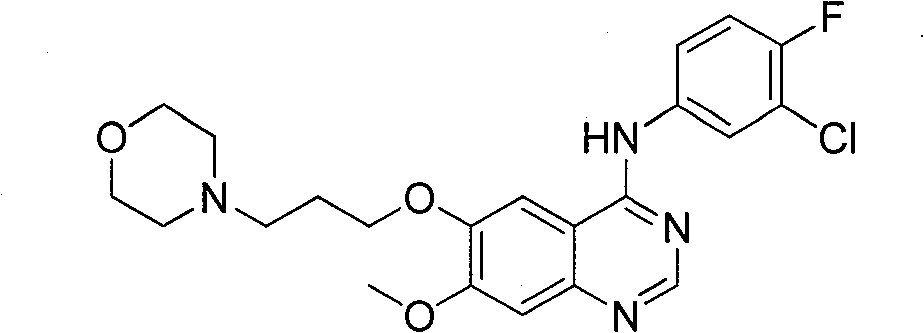
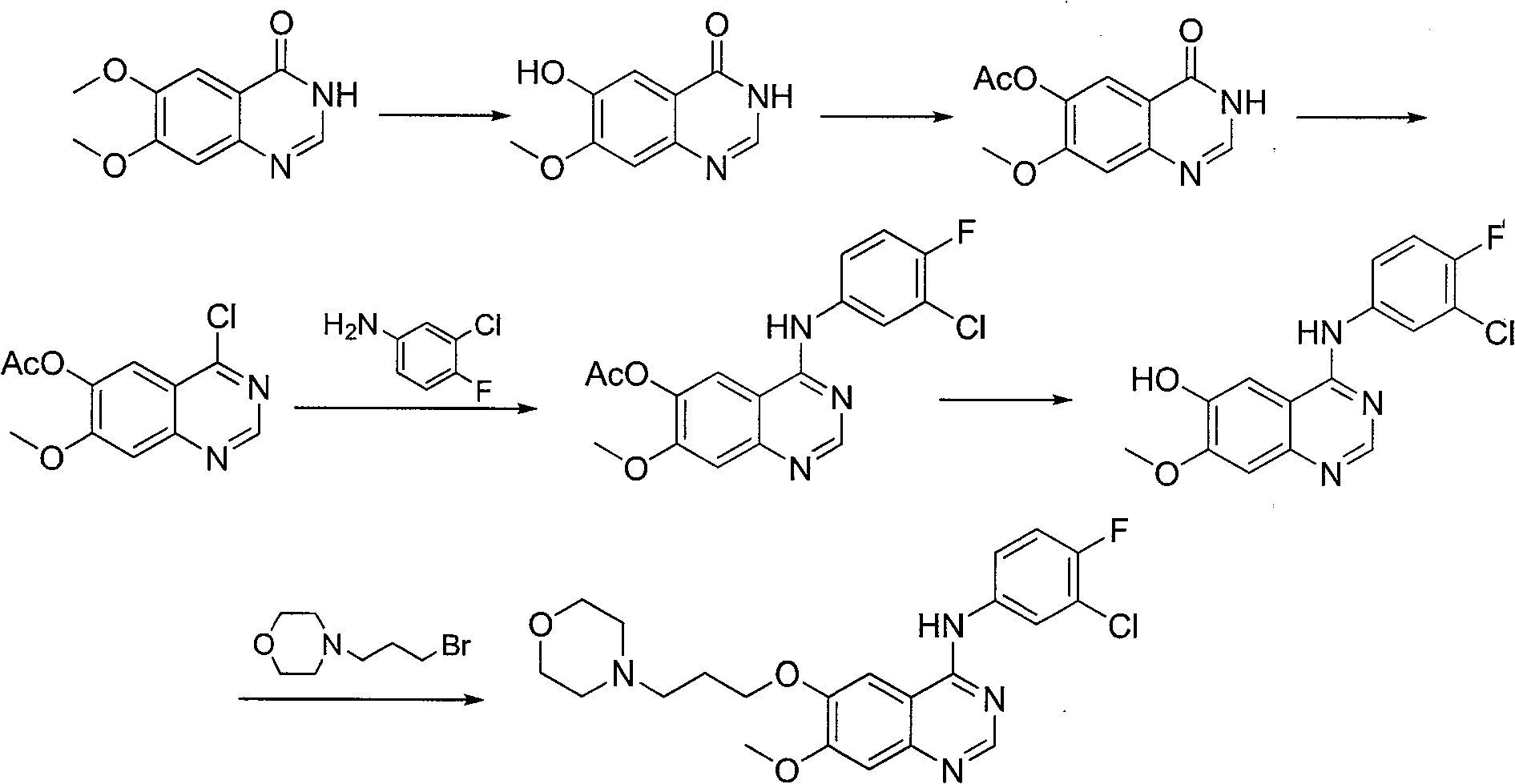
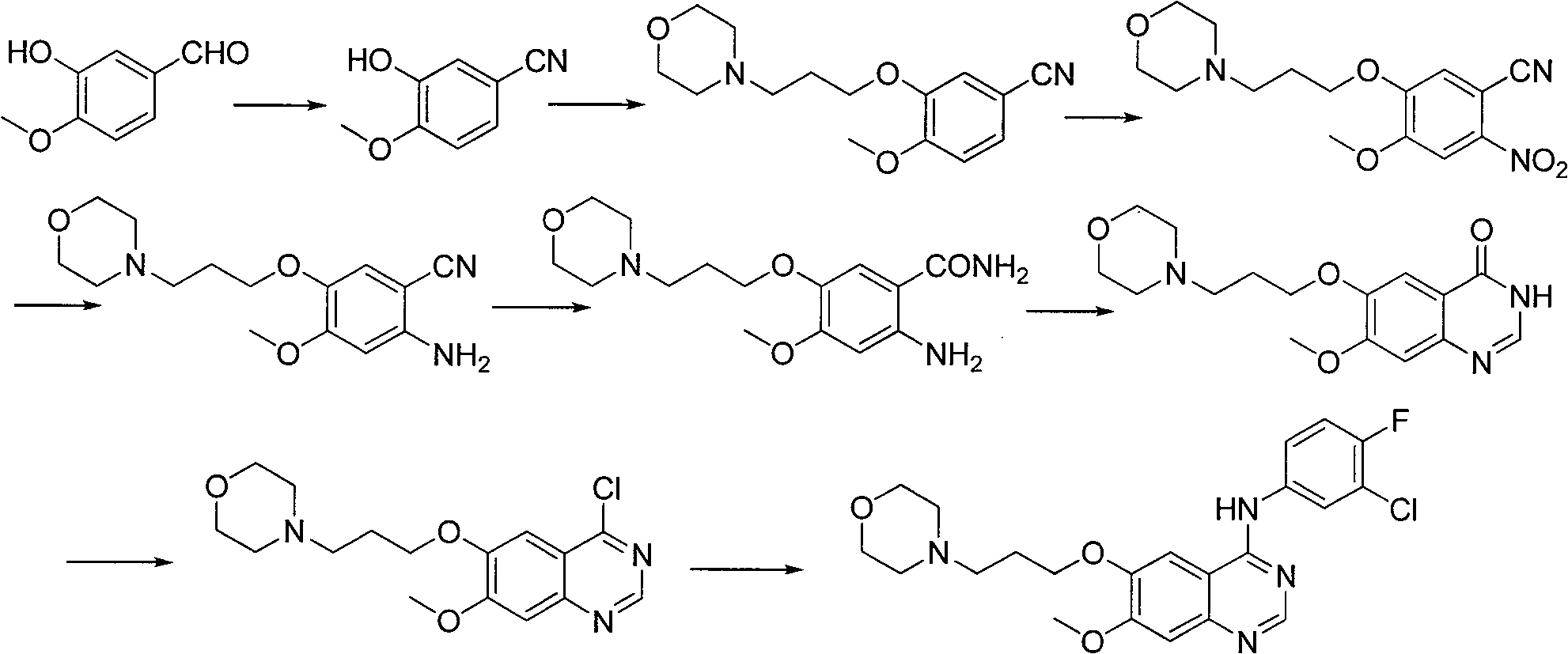
Method for preparing gefitinib
A technology of gefitinib and methoxy, which is applied in the field of medicinal chemistry, can solve the problems of many by-products, low yield, and many side reactions, and achieve the effects of less waste, high yield, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0048] The first step: the preparation of 3,4-dimethoxy-6-nitrobenzoic acid (3):
[0049] Place the 2L three-necked flask in an ice bath, and add 1100ml HNO to the bottle 3 (65%~68%), insert a thermometer and a mechanical stirring device, add 200g of 3,4-dimethoxybenzoic acid (2) in small batches under stirring, about 1h, after adding, continue to stir for 15min, and then Pour it into a container containing 10L of cold water under stirring. After adding, continue to stir for 10 minutes, filter, suck dry, then beat with 5L cold water for a few minutes, filter, and drain as much as possible. The crude product was dried in a vacuum oven at 50° C. for 48 hours to obtain 237 g of crude product. It was recrystallized from 95% ethanol to obtain 199 g of a yellow solid with a yield of 80%.
[0050] Second step: the preparation of 2-nitro-4-methoxy-5-hydroxybenzoic acid (4):
[0051] 199g of 3,4-dimethoxy-6-nitrobenzoic acid (3) was added to 1L of 20% NaOH solution, heated and stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com