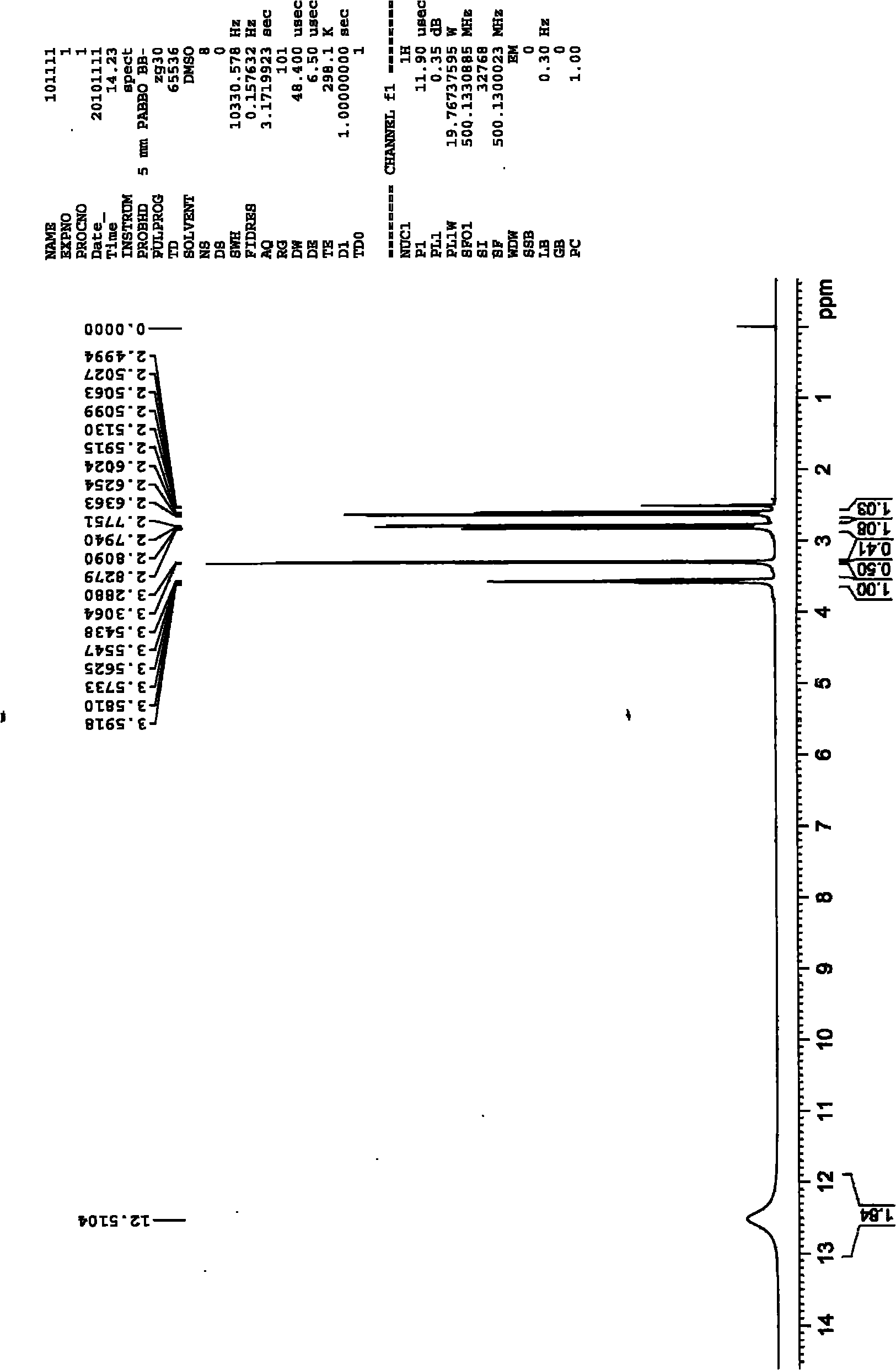
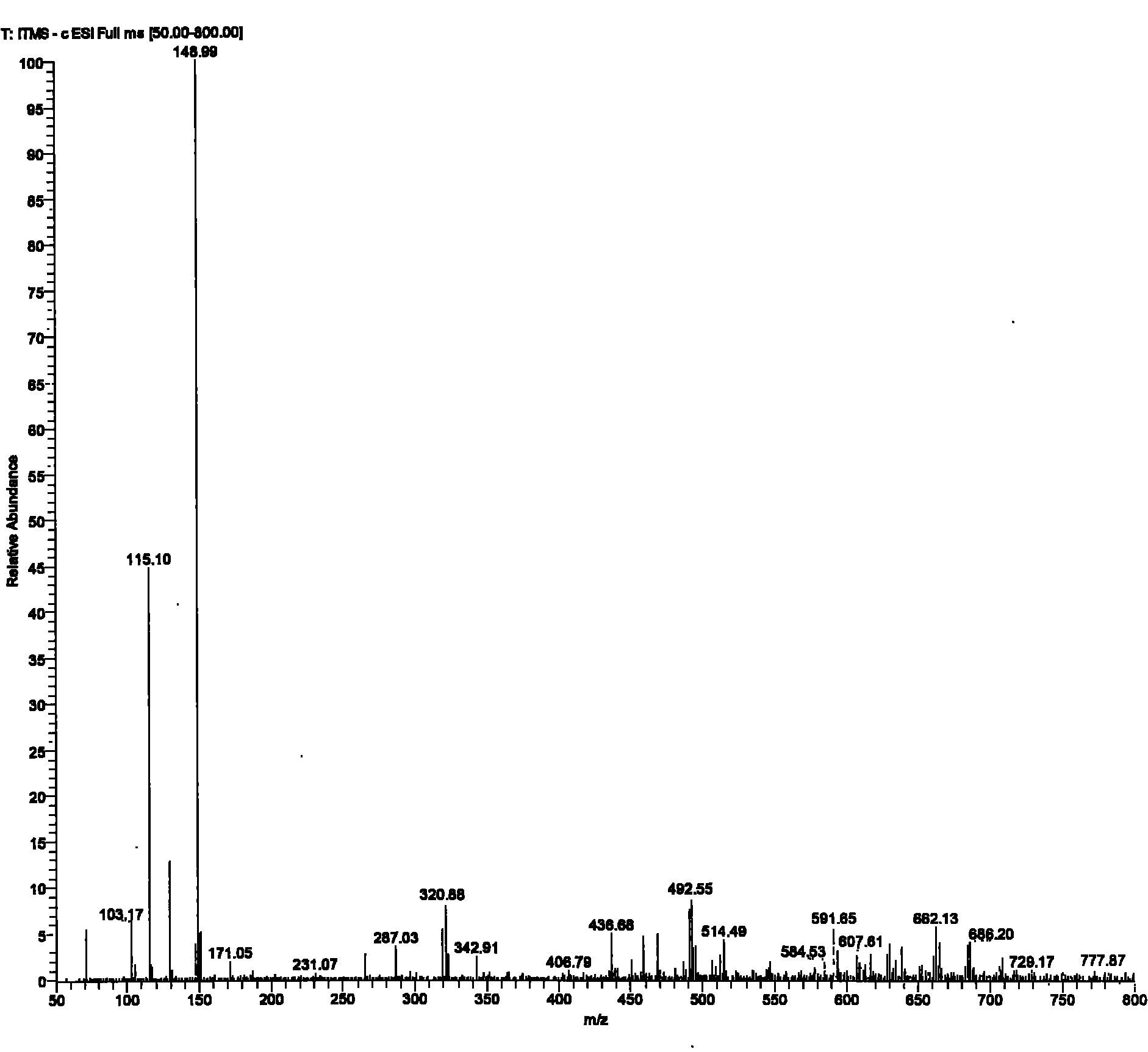
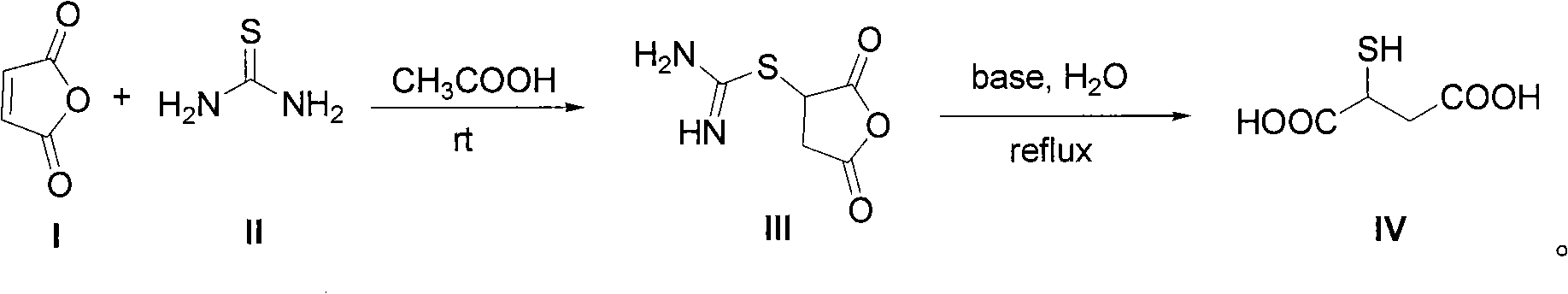
Method for synthesizing 2-mercaptosuccinic acid
The technology of a kind of mercaptosuccinic acid and its synthesis method is applied in the field of preparation of 2-mercaptosuccinic acid, which can solve problems such as unfavorable industrial large-scale production, inability to separate inorganic salts, unfavorable production costs, etc., and achieve easy industrialization Large-scale production, convenient operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] To a 250 mL three-necked flask equipped with mechanical stirring and a reflux condenser was added 24.6 g of maleic anhydride followed by 130 g of glacial acetic acid. After the solids were dissolved, 19.0 g of thiourea was added with stirring. The reaction system was stirred at room temperature for 12 hours. After filtration, the filter cake was washed twice with glacial acetic acid and water, respectively. After drying, 43.2 g of a white solid was obtained with a yield of 99%.
[0030] The obtained intermediate (III) was added to 120 g of an aqueous solution of 49.6 g of NaOH, and then heated under reflux for reaction for 4 hours. After the reaction system was cooled, it was acidified to pH 3 with 36%-38% concentrated hydrochloric acid. Part of the solvent was evaporated under reduced pressure to about 20 mL of remaining water, heated to reflux at 110° C. for 10 minutes, then cooled, filtered with suction, and washed with a small amount of cold water to obtain a whi...
Embodiment 2
[0034] To a 500 mL three-necked flask equipped with mechanical stirring and a reflux condenser was added 49.1 g of maleic anhydride followed by 250 g of glacial acetic acid. After the solids were dissolved, 38.0 g of thiourea was added with stirring. The reaction system was stirred at room temperature for 12 hours. After filtration, the filter cake was washed twice with glacial acetic acid and water, respectively. After drying, 86.2 g of a white solid was obtained with a yield of 99%.
[0035] The obtained intermediate (III) was added to 200 g of an aqueous solution of 99.0 g of NaOH, and then heated under reflux for reaction for 4 hours. After the reaction system was cooled, it was acidified to pH 3 with 36%-38% concentrated hydrochloric acid. Part of the solvent was evaporated under reduced pressure to about 40 mL of remaining water, continued to heat under reflux for 10 minutes, then cooled, filtered with suction, and washed with a small amount of cold water to obtain a ...
Embodiment 3
[0038] To a 1 L three-necked flask equipped with mechanical stirring and a reflux condenser was added 98.1 g of maleic anhydride followed by 500 g of glacial acetic acid. After the solids were dissolved, 76.0 g of thiourea was added with stirring. The reaction system was stirred at room temperature for 12 hours. After filtration, the filter cake was washed twice with glacial acetic acid and water, respectively. After drying, a white solid 170.6 was obtained in 98% yield.
[0039] The obtained intermediate (III) was added to 400 g of an aqueous solution of 195.9 g of NaOH, and then heated under reflux for reaction for 4 hours. After the reaction system was cooled, it was acidified to pH 3 with 36%-38% concentrated hydrochloric acid. Part of the solvent was evaporated under reduced pressure to about 120 mL of remaining water, heated to reflux for 10 minutes, then cooled, filtered with suction, and washed with a small amount of cold water to obtain a white solid and a pale yel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com