Preparation method and application of ruthenium and rhodium transition metal complex functional ionic liquid
A technology of ionic liquids and rhodium complexes, which is applied in the preparation of organic compounds, hydroxyl compounds, organic compound/hydride/coordination complex catalysts, etc., which can solve the cumbersome operation of product separation and purification, and limit the use of products , poor catalyst stability and other problems, to achieve the effect of good chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
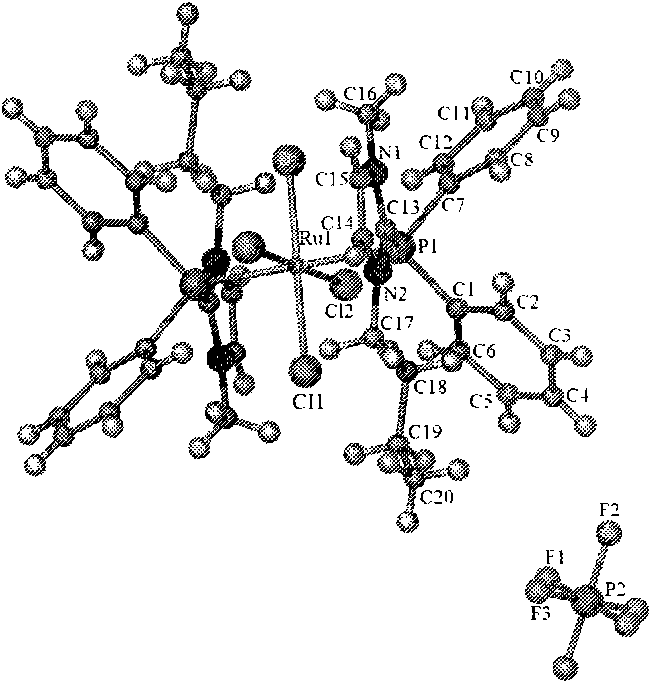
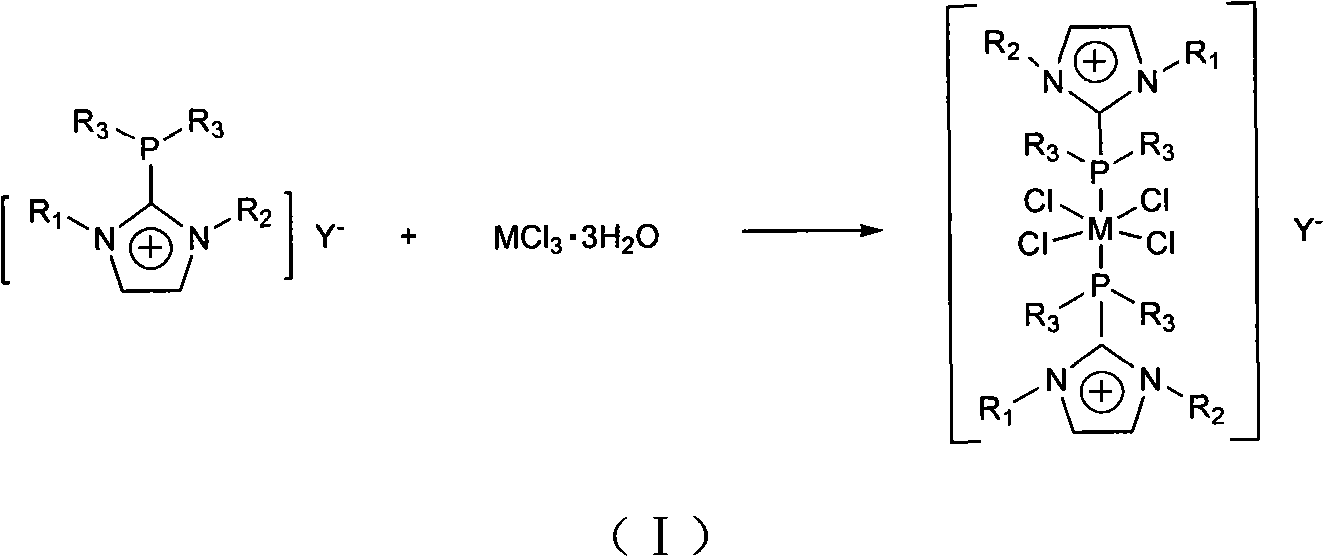
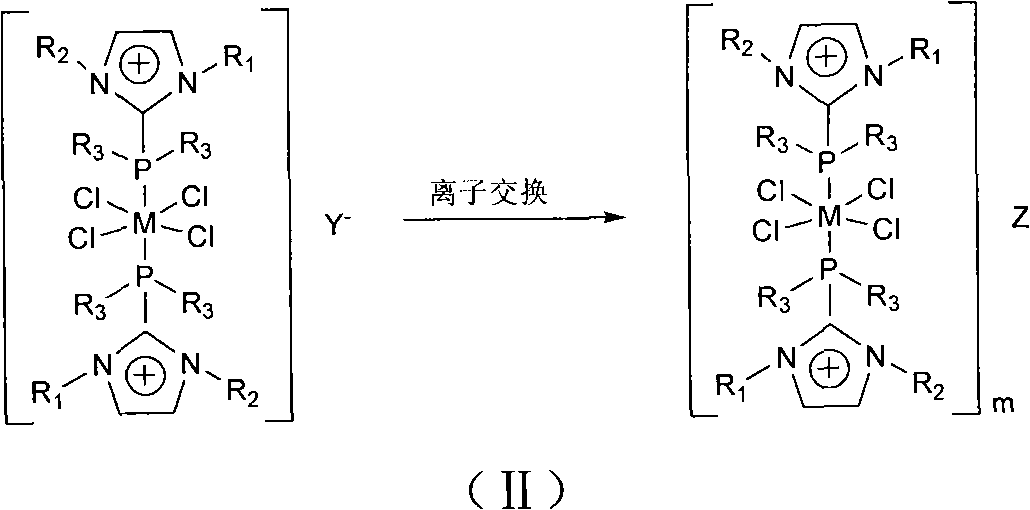
[0027] In a nitrogen atmosphere, dissolve 0.5g of ruthenium trichloride hydrate in 20mL of acetone, then add 3.4g of 1-butyl-2-diphenylphosphino-3-methylimidazolium hexafluorophosphate, and stir under reflux for 16h. The reaction solution was cooled to room temperature, and an orange solid was precipitated. The solid was collected, washed with methanol and diethyl ether, and finally vacuum-dried to obtain ruthenium complex functionalized ionic liquid 1 with a yield of 55%. The characterization results of 1 are as follows. FT-IR (Nicolet NEXUS 670, KBr tablet): 3737(w), 3447(w), 2960(s), 1620(w), 1395(m), 1260(m), 841(PF 6 - , s). 1 H NMR (Bruker Avance 500, DMSO): δ0.70(t, 3H, CH 2 CH 2 CH 2 CH 3 ), 1.03 (m, 2H, CH 2 CH 2 CH 2 CH 3 , 1.42(m, 2H, CH 2 CH 2 CH 2 CH 3 ), 3.53 (s, 3H, NCH 3 ), 4.20(t, 2H, CH 2 CH 2 CH 2 CH 3 ), 7.43 (m, 4H, CH 3 NCP (Ph 2 )N + ), 7.56 (m, 6H, CH 3 NCP (Ph 2 )N + ), 8.03(s, 1H, NC(H)C(H)N + ), 8.12(s, 1H, NC(H)C(H)N + )....
Embodiment 2
[0030] In a nitrogen atmosphere, dissolve 0.5g of ruthenium trichloride hydrate in 20mL of acetone, then add 3.0g of 1-butyl-2-diphenylphosphino-3-methylimidazolium tetrafluoroborate, and stir for 6h under reflux, The reaction solution was cooled to room temperature, and an orange solid was precipitated. The solid was collected, washed with methanol and ether, and finally vacuum-dried to obtain ruthenium complex functionalized ionic liquid 2 with a yield of 40%. FT-IR (Nicolet NEXUS 670, KBr tablet): 3729(w), 3440(w), 2969(s), 1628(w), 1387(m), 1253(m), 1046(BF 4 - , s). 1 H NMR (Bruker Avance 500, DMSO): δ0.72 (t, 3H, CH 2 CH 2 CH 2 CH 3 ), 1.03 (m, 2H, CH 2 CH 2 CH 2 CH 3 , 1.42(m, 2H, CH 2 CH 2 CH 2 CH 3 ), 3.53 (s, 3H, NCH 3 ), 4.20(t, 2H, CH 2 CH 2 CH 2 CH 3 ), 7.40 (m, 4H, CH 3 NCP (Ph 2 )N + ), 7.53 (m, 6H, CH 3 NCP (Ph 2 )N + ), 8.03(s, 1H, NC(H)C(H)N + ), 8.12(s, 1H, NC(H)C(H)N + ). 31 P NMR (δ, ppm): -29.0 (s, PPh 2 ), CHN elemental analy...
Embodiment 3
[0033] In a nitrogen atmosphere, 0.2 g of ruthenium complex functionalized ionic liquid 1 was dissolved in 30 mL of acetone, an excess of phosphotungstic acid was added, and stirred at room temperature for 24 h, an orange solid was precipitated. The solid was collected, washed with acetone and diethyl ether, and finally vacuum-dried to obtain ruthenium complex functionalized ionic liquid 3 with a yield of 81%. FT-IR (Nicolet NEXUS 670, KBr tablet): 3440(w), 2932(s), 1638(w), 1084(P-O a ), 982 (terminal W-O d ), 896 (W-O between octahedra b -W), 808 (W-O in octahedron c -W)cm -1 . 1 H NMR (Bruker Avance 500, DMSO): δ0.70(t, 3H, CH 2 CH 2 CH 2 CH 3 ), 1.05 (m, 2H, CH 2 CH 2 CH 2 CH 3 , 1.43(m, 2H, CH 2 CH 2 CH 2 CH 3 ), 3.53 (s, 3H, NCH 3 ), 4.21(t, 2H, CH 2 CH 2 CH 2 CH 3 ), 7.44 (m, 4H, CH 3 NCP (Ph 2 )N + ), 7.56 (m, 6H, CH 3 NCP (Ph 2 )N + ), 8.02(s, 1H, NC(H)C(H)N + ), 8.11(s, 1H, NC(H)C(H)N + ). 31 P NMR (δ, ppm): -14.4(s), -28.2(s, PPh 2 ), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com