Preparation method of alpha-acyloxy ether
A technology of acyloxyether and alkyl, applied in the field of organic synthesis, can solve problems such as cumbersome steps, and achieve the effects of avoiding metal pollution, reducing pollution, and high compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
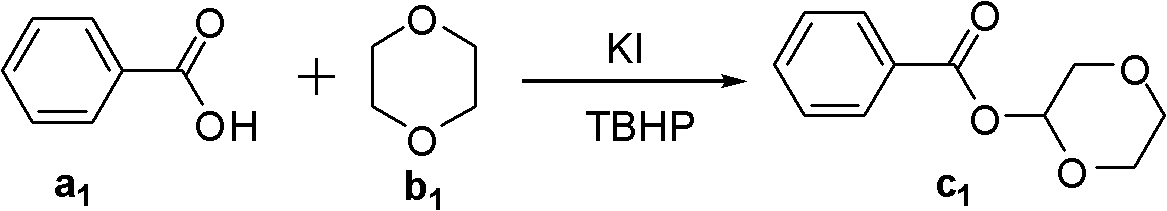
[0023]
[0024] Load KI (0.1mmol) successively in the reaction bottle, compound a 1 (0.5 mmol), compound b 1 (2 mL), TBHP (2.2 equiv). Then the system was stirred in the air at 100°C for about 2 hours, then a saturated sodium sulfite solution was added to quench the reaction, extracted with ethyl acetate (2mL×3), then adsorbed with 100-200 mesh silica gel, and then passed through a 300-400 mesh The product c was obtained by elution of silica gel column 1 , the yield was 97%. 1 H NMR (CDCl 3 , 400MHz): δ3.66-3.70(m, 1H), 3.82-3.84(m, 2H), 3.89-3.90(m, 2H), 4.21-4.24(m, 1H), 6.10(t, J=2.0Hz , 1H), 7.44-7.48(m, 2H), 7.57-7.61(m, 1H), 8.12-8.14(m, 2H); 13 C NMR (CDCl 3 , 100MHz): 61.6, 66.0, 67.7, 89.7, 128.3, 129.6, 129.7, 133.3, 165.1; ESI-MS (C 11 h 12 o 4 Na): 231; IR (KBr, cm -1 ): 1726. The above detection data confirmed that the target product was obtained.
Embodiment 2
[0026]
[0027] The reaction bottle is filled with I 2 (0.1 mmol), compound a 2 (0.5 mmol), compound b 1 (2 mL), hydrogen peroxide (2.2 equivalents). After stirring for about 8 hours at 80°C in air, add saturated sodium sulfite solution to quench the reaction, extract with ethyl acetate (2mL×3), then absorb with 100-200 mesh silica gel, and then pass through a 300-400 mesh silica gel column. Washed product c 2 , the yield was 97%. 1 H NMR (CDCl 3 , 400MHz): δ3.68-3.72(m, 1H), 3.82-3.95(m, 4H), 4.23-4.29(m, 1H), 6.11(t, J=1.8Hz, 1H), 7.16-7.20(m , 1H), 7.41-7.45(m, 1H), 7.92-7.95(m, 1H), 8.01-8.04(m, 1H); 13 C NMR (CDCl 3 , 100MHz): δ61.6, 65.8, 67.4, 90.4, 94.1, 127.8, 131.2, 132.9, 134.0, 141.3, 164.7; ESI-HRMS: calcd.for C 11 h 11 NaIO 4 , 356.9600; found, 356.9592; IR (KBr, cm -1 ): .1699. The above detection data confirmed that the target product was obtained.
Embodiment 3
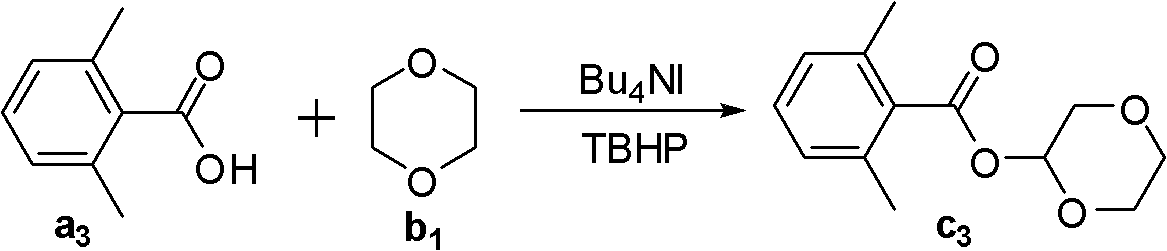
[0029]
[0030] Fill the reaction bottle with Bu 4 NI (0.1 mmol), compound a 3 (0.5 mmol), compound b 1 (2 mL), TBHP (2.2 equiv). Then the system was stirred in the air at 50°C for about 18 hours, then a saturated sodium sulfite solution was added to quench the reaction, extracted with ethyl acetate (2mL×3), then adsorbed with 100-200 mesh silica gel, and then passed through a 300-400 mesh The product c3 was obtained by elution on a silica gel column with a yield of 71%. 1 HNMR (CDCl 3 , 400MHz): δ2.37(s, 6H), 3.67-3.70(m, 1H), 3.78-3.82(m, 2H), 3.87-3.88(m, 2H), 4.11-4.21(m, 1H), 6.13 (t, J=2.0Hz, 1H), 7.04-7.05(m, 2H), 7.19-7.22(m, 1H); 13 C NMR (CDCl 3, 100MHz): δ19.6, 61.7, 65.9, 67.6, 89.9, 127.5, 129.4, 133.1, 134.8, 168.6; ESI-MS (C 13 h 16 NaO 4 ): 259; IR (KBr, cm -1 ): 1733. The above detection data confirmed that the target product was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com