Preparation method of sodium ferulate freeze-dried powder injection
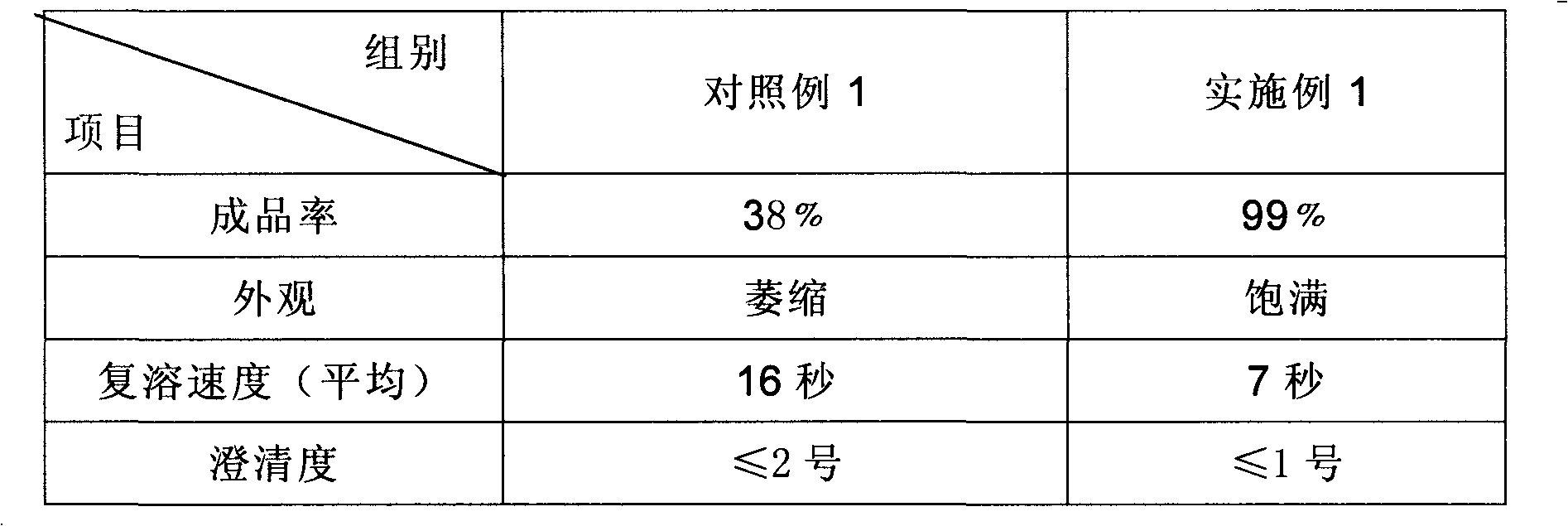
A technology of freeze-dried powder injection and sodium ferulate, which is applied in the direction of freeze-dried transportation, anti-toxic agents, anti-inflammatory agents, etc. It can solve the problems of appearance collapse, poor frozen shape, difficult control of resolubility and clarity, and achieve stability Strong resistance, good resolubility, and loose texture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
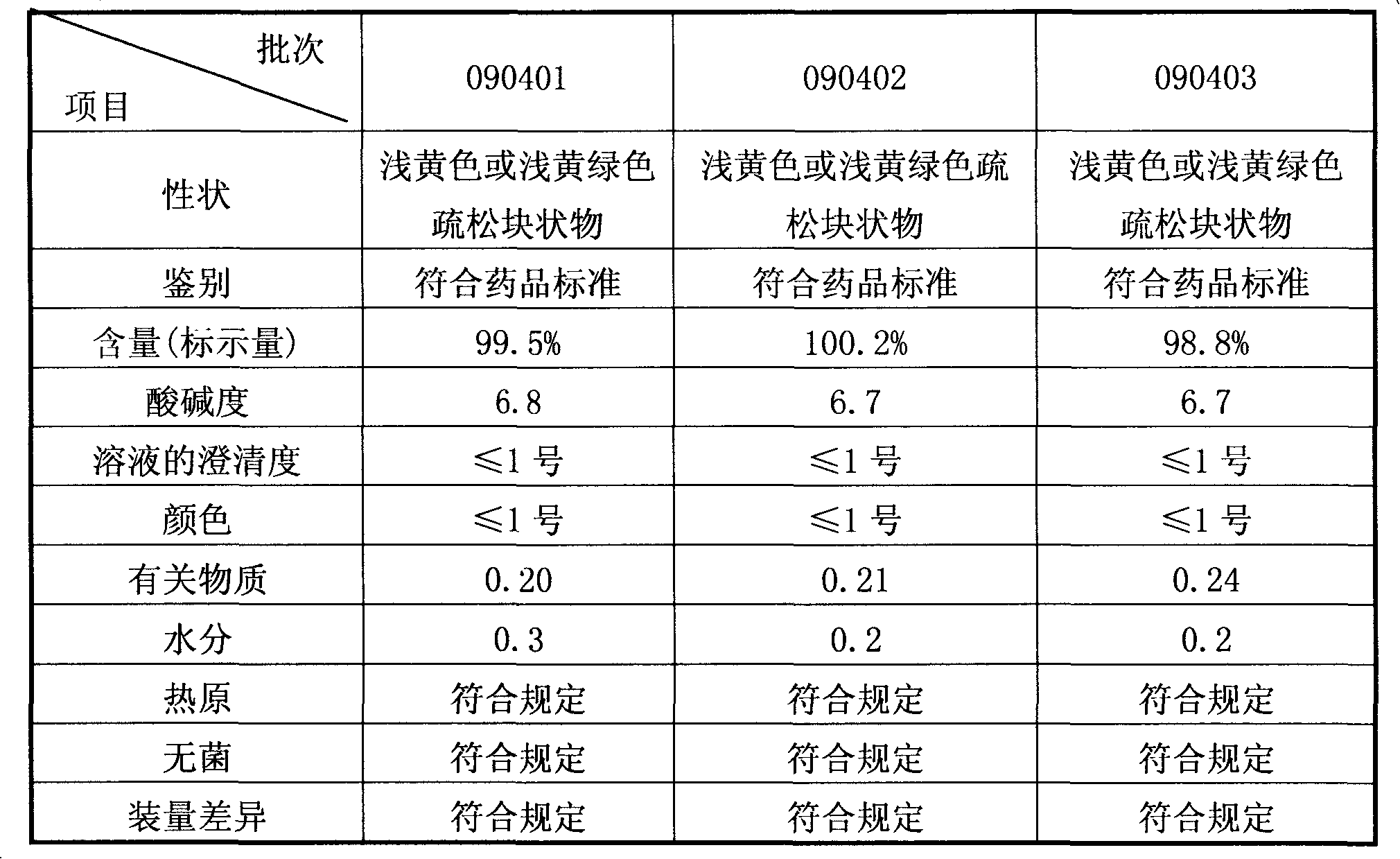
[0017] Sodium ferulate freeze-dried powder injection 0.15g sodium ferulate in water for injection per 2ml.
[0018] making process:
[0019] a. Add about 80% pre-prepared volume of water for injection into the batching tank, control the water temperature at 20°C, and operate in the dark. Pour nitrogen into the batching tank, add the prescribed amount of sodium ferulate powder, and stir while adding to make it dissolve. During the process, check the pH value to make it between 6.8 and 7.2. Add 0.1% (g / ml) activated carbon for needles to the above solution, and make up the volume with the remaining water for injection. After stirring for 30 minutes, decarbonize by adding filter paper through a plate and frame filter press. The laboratory takes samples, checks the pH value, and performs sterile pressure filtration after passing the test. Filter through a 0.22μm microporous membrane filter in the 100-class clean packaging room, fill the liquid medicine according to the liquid m...
Embodiment 2
[0030] (a) Add about 80% pre-prepared volume of water for injection in the batching tank, control the water temperature at 15°C, add the original powder of sodium ferulate, and check the pH value to make it 6.8; add 0.2% (g / ml) Activated carbon, use the remaining water for injection to make up the volume, stir to decolorize and depyrogenate, filter, test the content, and pack;
[0031] (b) freeze-dry according to the following steps:
[0032] (1) Pre-freezing: place the product on the partition of the freeze-drying box, cool the product to -45°C, and keep it warm for 6 hours;
[0033] (2) Sublimation: raise the temperature of the product to -6°C and keep it warm for 2 hours, then lower it to -40-45°C, and cool the condenser to below -45°C, vacuumize, control the vacuum degree at 10-20pa, and keep it warm for 3 hours ;
[0034] (3) Analytical drying: raise the temperature by 5°C every 1.5 hours until the temperature of the product is about -30°C, then slowly increase the temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com