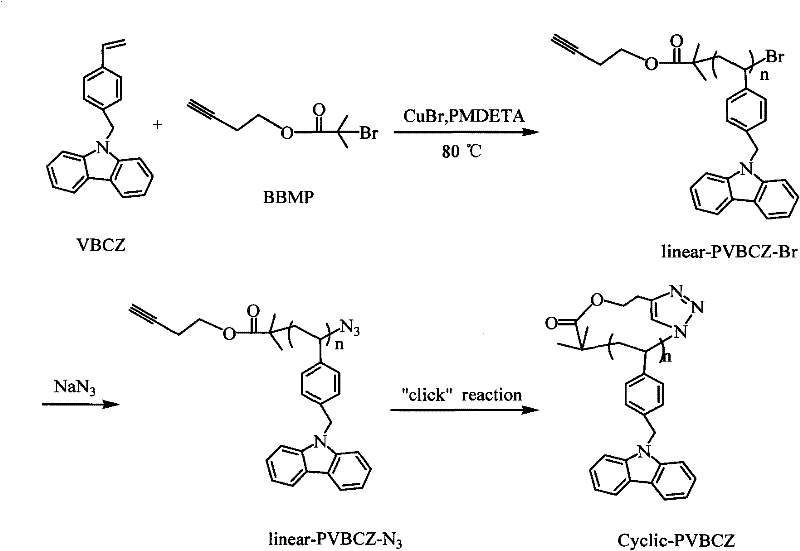
Cyclic polymer and preparation method thereof
A cyclic polymer and cyclic technology, applied in the field of high molecular polymers, can solve the problems of difficulty and limitation in the synthesis of linear polymers, and achieve the effects of convenient separation and application, good thermal stability, and enhanced fluorescence of substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment one: preparation of cyclic polystyrene carbazole, such as figure 1 As shown, the synthesis of cyclic polystyrene carbazole is divided into three main steps: ① Use styrene carbazole as a monomer and synthesize a bifunctional linear polystyrene carbazole with α-alkynyl-ω-bromine by ATRP. azole linear-PVBCZ-Br(a); ② use sodium azide to convert the terminal bromine of linear-PVBCZ-Br(a) into an azido group to obtain linear-PVBCZ-N 3 (b); ③ Obtain cyclic-PVBCZ (c) through intramolecular "click" cyclization reaction; the number average molecular weight and molecular weight distribution of the polymer are shown in Table 1.
[0031] Specifically include the following steps:
[0032] 1. Add 2.0 g styrene carbazole (VBCZ) (7.1×10 -3 mol), 30 μL initiator BBMP (1.8mmol), 109 μL ligand PMDETA (5.3mmol), 2.5×10 -2 g catalyst CuBr and 15mL solvent anisole, and then the reaction tube was closed-melted through the freezing-vacuum cycle three times, and placed in an oil ba...
Embodiment 2
[0038] Embodiment two: analyze the linear and cyclic polystyrene carbazole in embodiment one.
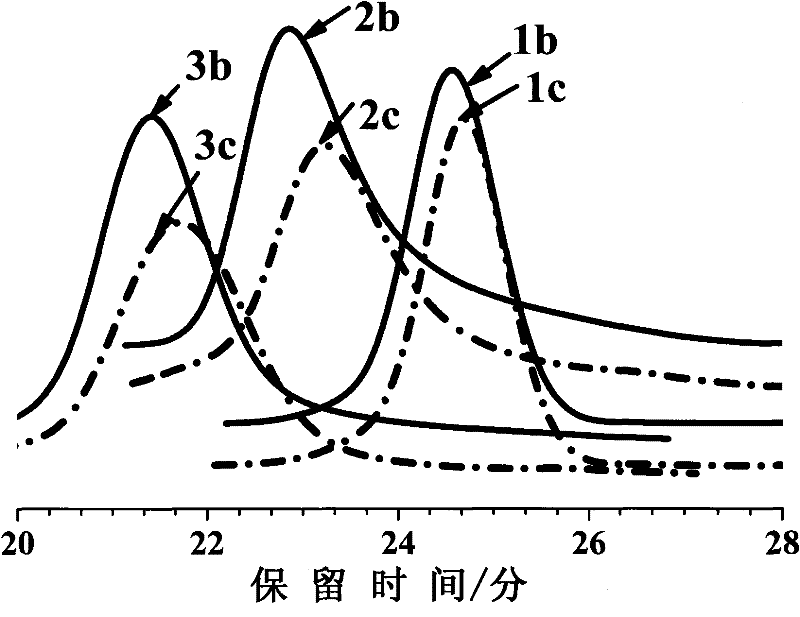
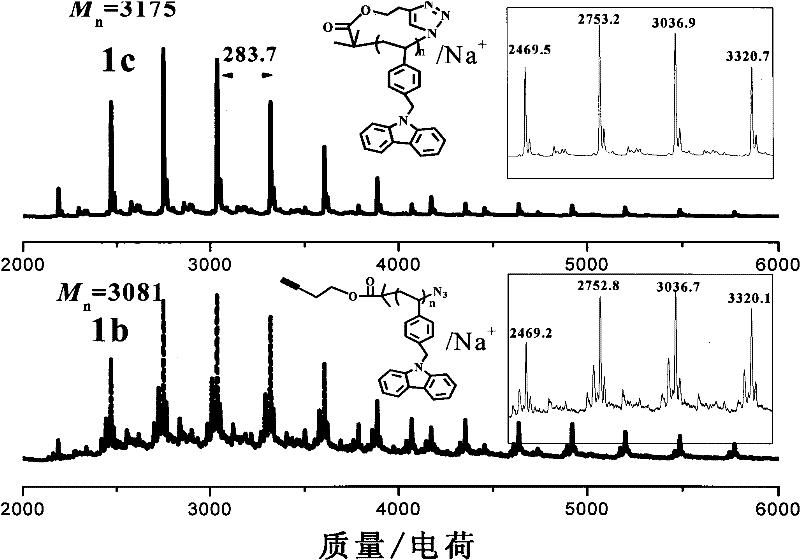
[0039] For the elution curves of GPC of linear and cyclic polystyrene carbazoles see figure 2 , it can be seen that the retention time of the three cyclic-PVBCZ elution peaks is longer than that of the corresponding linear polymer. Depend on image 3 It can be seen from the MALDI-TOF spectrum that the actual molecular weights of ring and line polymers are actually the same. According to the report of Kramers et al. and Zimm et al. (referring to: Kramers, H.A.J.Chem.Phys.1946,14,415-424; Zimm, B.H.; Stockmayer, W.H.J.Chem.Phys.1949,17,1301-1314), This phenomenon is known to be due to the smaller hydrodynamic radius of the cyclic polymer than the linear precursor.
[0040] From the FT-IR spectrum ( Figure 4 ) can also be seen, the linear polymer at 2090cm -1 The azide peak at and 3300cm -1 The alkynyl peaks at have disappeared after the cyclization reaction, proving that there...
Embodiment 3
[0042] Example 3: Thermal performance test of Cyclic-PVBCZ and Linear-PVBCZ
[0043] In Marzio's report (see: Edmund A. Di Marzio * and Charles M. Guttman Macromolecules 1987, 20, 1403-1407), since the three-dimensional structure of the ring is much smaller than that of a line of the same length, the conformational entropy ΔS of a cyclic polymer is always less than that of a linear polymer of the same molecular weight. ΔG=ΔH-TΔS, at the time of glass transition, in order to make ΔG equal to 0, under the same ΔH, the ΔS of the linear polymer is greater than that of the cyclic polymer, so T is smaller than that of the cyclic polymer. That is to say, in the case of the same molecular weight, the glass transition temperature (T g ) is always higher than linear. This phenomenon was also observed in our analysis of styrene carbazole polymers using differential scanning calorimetry. As shown in Table 2, the T of cyclic-PVBCZ(c) g It is higher than the corresponding linear-precurs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com