Prokaryotic expression vector and application thereof
A prokaryotic expression and carrier technology, applied in the field of bioengineering, can solve the problems of loss of target protein, failure to effectively increase the yield of target protein, effective folding of soluble expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
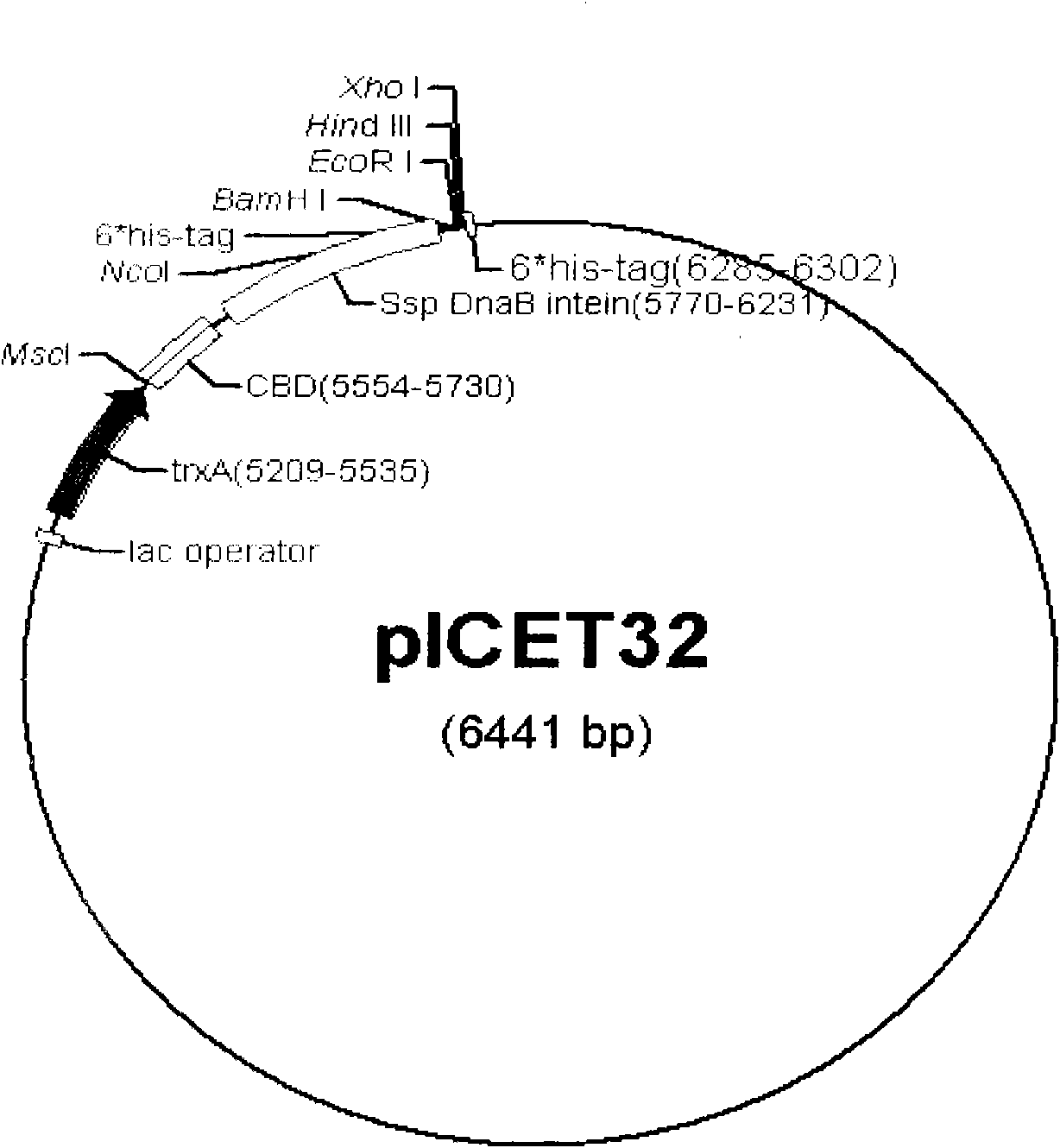
[0018] The construction of embodiment 1 expression vector pICET32
[0019] The starting vector is pET32a, which has a high-efficiency T7 promoter and contains thioredoxin TrxA. It is one of the commonly used vectors to promote high-efficiency protein soluble expression. Design upstream primer DP1: 5'-CA TGGCCA TATGAAAATCGAAGAAGGTAAAC-3' (the underlined part in italics is the restriction endonuclease Msc I site) and downstream primer DP2: 5'-GTCCATGGCCGTTGTGTACAATGATGTCATTC-3' were amplified from the expression vector pTWIN1 (product of NEB Company) with pfu enzyme to obtain several The nucleotide sequence encoding the butyrin-binding domain (CBD) and the intein mini SspDnaB was used as a template to amplify the second-round product with the upstream primer DP1 and the newly designed primer 5'-GTACACAACGGCCATGGACATCATCATCATCATCATGGATCC-3'. A restriction endonuclease NcoI cutting site and a 6×his tag coding sequence were introduced through this round of amplification. Using t...
Embodiment 2
[0021] Example 2 Expression of hookworm anticoagulant peptide AcaNAP10 by pICET32 and its isolation and purification
[0022] According to the coding sequence of hookworm anticoagulant peptide AcaNAP10 (SEQ ID NO.1), primers were designed to amplify the coding nucleic acid sequence from the adult cDNA of hookworm, and the primer sequence was designed as: N10-3: 5'-GAGGATCCAATCCAAGCTGTGGTGAG-3' (including Dicer site BamH I); N11-2: 5'-CGAAGCTTGGTCATTTTCTATTAGGG-3' (contains endonuclease site Hind III). After the PCR product was recovered and purified, it was digested with BamH I and Hind III, and then ligated overnight with the expression plasmid pICET32 that had undergone the same double digestion. The ligation product was transformed into E.coli DH5α competent cells and cultured in a medium containing ampicillin. The recombinant cloned plasmid identified by PCR and sequencing was named AcaNAP10 / pICET32. The recombinant expression plasmid was transformed into Escherichia col...
Embodiment 3
[0024] Example 3 Expression of serine protease inhibitor AduKuI4 and its separation and purification
[0025]AduKuI4 is a Kunitz-type serine protease inhibitor isolated from Ancylostoma duodenale by the inventor's research group. It contains 3 pairs of disulfide bonds. The amino acid sequence of its mature peptide is: RNPHRKGRCGDDPAETGGECPDPETKYTYKFGDCHEVKYCGEQETRNLFDSYEKCSGKCVIF. According to the mature peptide coding sequence of AduKuI4, the upstream primer: 5'-TAGGATCCCGCAATCCTCACAGAAAG-3' and the downstream primer: 5'-CCAAGCTTAGAAGATCACGCACTTTCC-3' were designed, and the mature peptide coding sequence was amplified from the adult cDNA of Ancylostoma duodenale. The amplified product was ligated into the expression vector to successfully construct the pICET32 / AduKuI4 expression plasmid. The plasmid was transformed into Escherichia coli BL21(DE3), induced and cultured, the cells were collected by centrifugation, and the supernatant was collected after sonication, and added wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com