Medicinal composition solution and preparation method and use thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as degradation and difficult preparation, and achieve the effects of inhibiting lipid peroxidation, being difficult to degrade, and stabilizing exposure to light.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
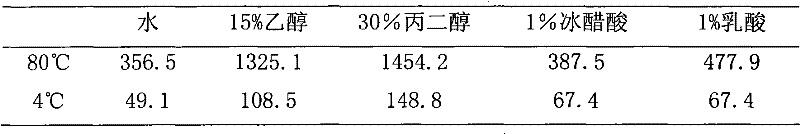
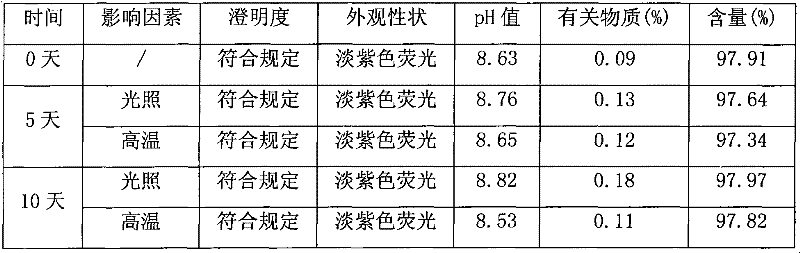
Image
Examples
Embodiment 1
[0025] In a nitrogen environment at 70°C, the antioxidant and L-arginine are dissolved in water according to the following weight ratio, and then Terisravone is added for dissolving to make Terreravone pharmaceutical composition solution, which The weight ratio is:
[0026] Teri Rabone 3g
[0027] L-Arginine 6g
[0028] Sodium thiosulfate 0.5g
[0029] Add water for pharmaceuticals to 1000ml
Embodiment 2
[0031] According to the following weight ratio:
[0032] Teri Rabone 3g
[0033] L-Arginine 6g
[0034] Sodium thiosulfate 0.5g
[0035] Add water for injection to 800ml
[0036] Add 0.5 g of sodium thiosulfate to 800 ml of water for injection at 80°C under nitrogen gas for 10 minutes, stir to dissolve, then add 6 g of L-arginine, and stir until completely dissolved. Add 3 g of Teriravone and stir until completely dissolved to obtain the pharmaceutical composition solution.
[0037] Add 1 g of activated carbon to the above-mentioned pharmaceutical composition solution, stir for 10 minutes, keep it at 70° C. for 20 minutes, and adjust the total amount to 1000 ml. The resulting solution was filtered with a membrane filter (0.45 μm in pore size) under a nitrogen atmosphere, and the pH was adjusted to 8.6 with hydrochloric acid. After the solution was clear and qualified, it was filled in an ampoule with a volume of 5 ml, replaced with nitrogen to remove air, and sterilized af...
Embodiment 3
[0044] According to the following weight ratio:
[0045] Teri Rabone 3g
[0046] L-Arginine 6g
[0047] Sodium thiosulfate 1g
[0048] Add water for injection to 800ml
[0049] Add 1.0 g of sodium thiosulfate to 800 ml of water for injection at 80° C. under nitrogen gas for 10 minutes, stir to dissolve, then add 6 g of L-arginine, and stir until completely dissolved. Add 3 g of Teriravone and stir until completely dissolved to obtain the pharmaceutical composition solution.
[0050] Add 1 g of activated carbon to the above pharmaceutical composition solution, stir for 10 minutes, keep it at 65° C. for 20 minutes, and adjust the total amount to 1000 ml. The resulting solution was filtered with a membrane filter (0.45 μm in pore size) under a nitrogen atmosphere, and the pH was adjusted to 8.6 with hydrochloric acid. After the solution was clear and qualified, it was filled in an ampoule with a volume of 5 ml, replaced with nitrogen to remove air, and sterilized after fusing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com