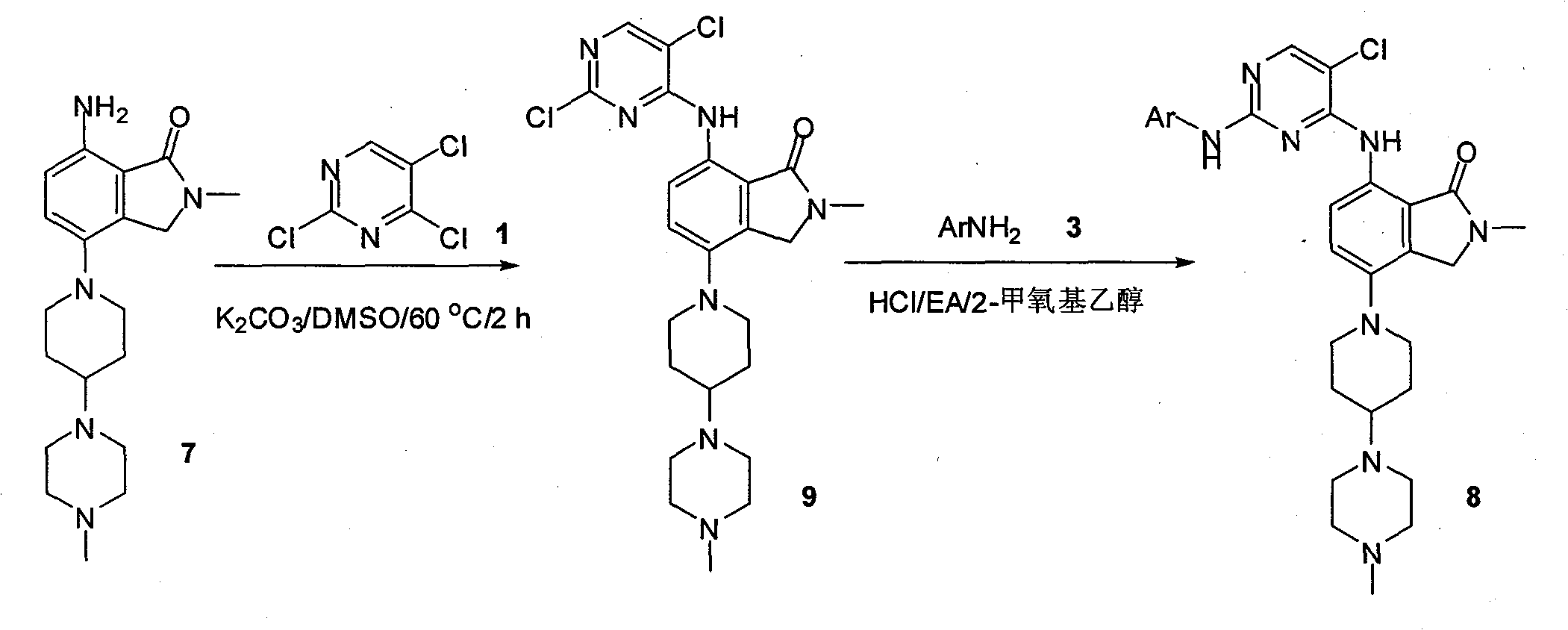
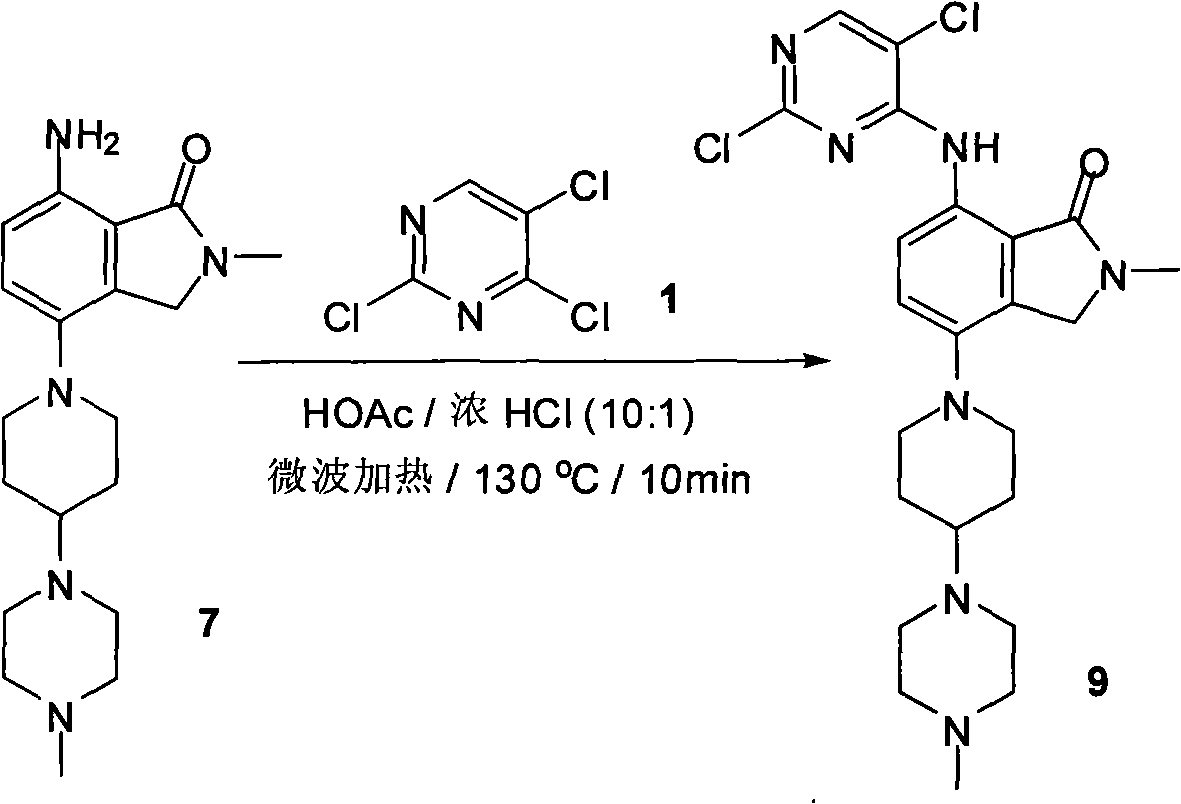
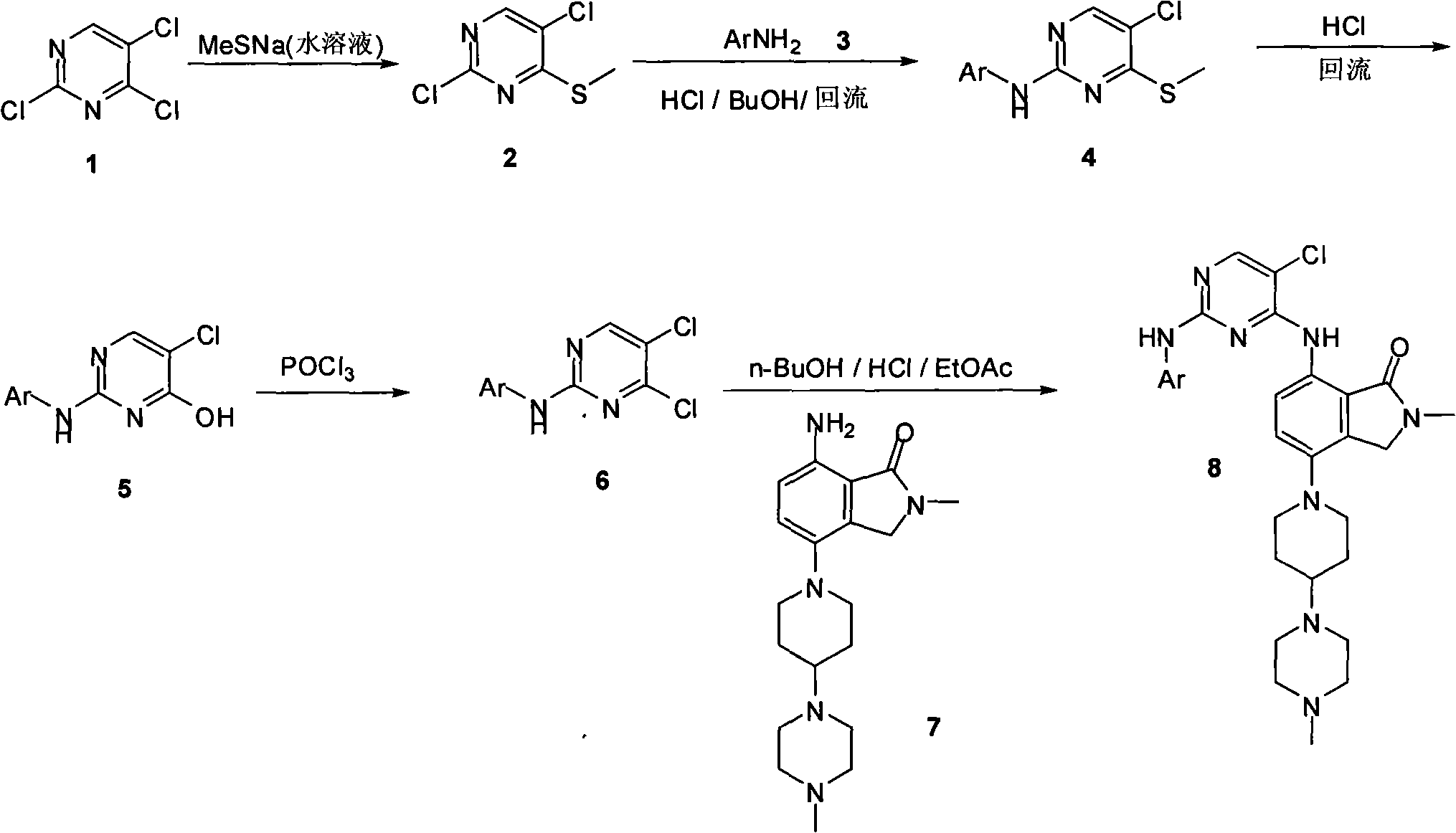
Synthesizing method of 2-methyl-7-(substituted pyrimidine-4-amino)-4-(substituted piperazine-1-base) piperidine-1-base) isoindoline-1-ketone and intermediate thereof
A synthesis method and technology of isoindoline are applied in the directions of drug combination, organic chemistry, antitumor drugs, etc., to achieve the effects of simple post-processing and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020]
[0021] 1.2, the synthetic technique of 5-dichloro-4-methylthiopyrimidine 2
[0022] 2,4,5-Trichloropyrimidine 1 (80g, 0.44mol) was dissolved in tetrahydrofuran (1000mL), and then a 21% by weight aqueous sodium methylthiolate solution (150g, 0.45mol) was slowly added dropwise to the reaction in the liquid. After the dropwise addition was completed, the reaction solution was stirred at room temperature for 24 hours. The reaction solution was poured into 200 mL of water. The mixture was extracted three times with 1 L of ethyl acetate. The combined organic phases were dried over anhydrous sodium sulfate. The desiccant was filtered, and the filtrate was concentrated to dryness to obtain 80g of pure product 2,5-dichloro-4-methylthiopyrimidine 2 (yield: 93%)
[0023] H NMR spectrum 1 H-NMR (DMSO, 400MHz): δ8.19 (s, 1H), 2.58 (s, 3H); MS (ESI) m / e (M+H + )195.
[0024] 2.2, The synthetic technique of 5-dichloro-4-methylthiopyrimidine 2
[0025] 2,4,5-Trichloropyrim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com