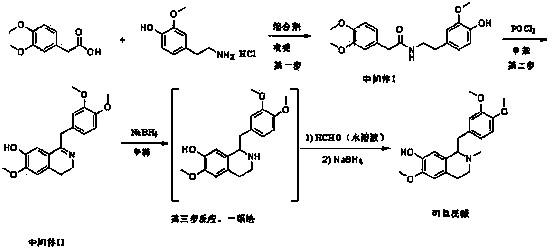
Synthetic method for codamine
The technology of a condensing agent and an intermediate is applied in the field of preparing ketamine, which can solve problems such as high separation difficulty, and achieve the effects of high separation difficulty, high identification difficulty and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 196 mg of 3,4-dimethoxyphenylacetic acid (purchased from Bailingwei Technology Co., Ltd.) and 245 mg of 4-hydroxy, 3-methoxyphenethylamine hydrochloride (purchased from Matrix Scientifi) and 569 mg HBTU (purchased from Bailingwei Technology Co., Ltd.) was added to 3 mL of pyridine, and then stirred at room temperature for 6 hours. Add 10 mL of ethyl acetate, wash with ammonium chloride aqueous solution and NaCl aqueous solution for 2-3 times to extract, and obtain the organic phase; dry the organic phase with anhydrous sodium sulfate, spin off the ethyl acetate to obtain intermediate I ( 350 mg, crude product, LCMS purity 92%)). Carry out NMR test to intermediate I, 1 H NMR (300 MHz, CDCl 3 ) δ 6.77 (dd, J = 8.3, 4.9 Hz, 2H), 6.69-6.64 (m, 2H), 6.58 (d, J = 1.9 Hz, 1H), 6.49 (dd, J = 8.0, 1.9 Hz, 1H) , 3.88 (s, 3H), 3.83 (d, J = 1.3 Hz, 6H), 3.46 – 3.38 (t, J = 6.9 Hz, 2H), 2.80 (s, 2H), 2.66 (t, J = 6.9 Hz, 2H). ESI 346 (M+1) + .
[0030] Add 350 mg of Intermedia...
Embodiment 2
[0033] 196 mg of 3,4-dimethoxyphenylacetic acid (purchased from Bailingwei Technology Co., Ltd.) and 225 mg of 4-hydroxy, 3-methoxyphenethylamine hydrochloride (purchased from Matrix Scientifi) 493 mg of HBTU (purchased from Bailingwei Technology Co., Ltd.) was added to 3 mL of pyridine, and then stirred at room temperature for 3 hours. Add 10 mL of ethyl acetate, wash with ammonium chloride aqueous solution and NaCl aqueous solution for 2-3 times, the organic phase is dried with anhydrous sodium sulfate, spin off ethyl acetate to obtain intermediate I (340 mg, crude product, LCMS purity 90 %).
[0034] Add 340mg of intermediate I to 3mL of toluene, then add 0.8 mL of phosphorus oxychloride to obtain a reaction solution; heat the reaction solution to 110°C and stir for 2 hours, then cool it, spin off toluene and excess phosphorus oxychloride , add a little ice-water mixture, adjust the pH value to 8-9 with sodium carbonate aqueous solution, then extract 2-3 times with 10 mL o...
Embodiment 3
[0037] 196 mg of 3,4-dimethoxyphenylacetic acid (purchased from Bailingwei Technology Co., Ltd.) and 205 mg of 4-hydroxy, 3-methoxyphenethylamine hydrochloride (purchased from Matrix Scientifi) and 455 mg of HBTU (purchased from Bailingwei Technology Co., Ltd.) was added to 3 mL of pyridine, and then stirred at room temperature for 3 hours. Add 10 mL of ethyl acetate, wash with ammonium chloride aqueous solution and NaCl aqueous solution 2-3 times, the organic phase is dried with anhydrous sodium sulfate, spin off ethyl acetate to obtain intermediate I (345 mg, crude product, LCMS purity 91%), directly used in the second step reaction.
[0038] Add 345mg of intermediate I to 3mL of toluene, and then add 0.5mL of phosphorus oxychloride to obtain a reaction solution; the reaction solution is heated to 110°C and stirred for 2 hours, then cooled, and the toluene and excess phosphorus oxychloride are removed by rotation. Add a little ice-water mixture, adjust the pH value to 8-9 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com