The purification method of 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroisoquinoline salt
A technology of methoxybenzyl and purification method, which is applied in the field of purification of salt formation of 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroisoquinoline , to achieve the effect of convenient and safe process, less waste and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
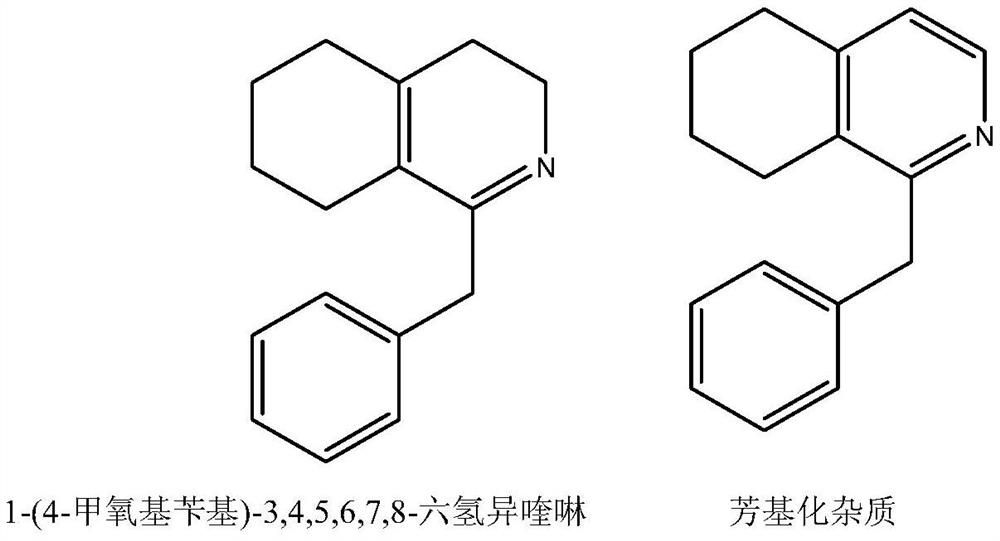
[0014] Add 150 milliliters of xylene, 110 grams of N-(2-(cyclohexenylethyl)-2-(4-methoxyphenyl)acetamide to the three-necked flask. Be warmed up to 85-90 ° C and controlled at this temperature 89.6g of phosphorus oxychloride was added dropwise, kept at 85 to 90°C for 3 hours, and cooled to room temperature. Concentrated under reduced pressure below 70°C, added 80ml of 80% methanol aqueous solution for 30min, then added 320ml of aqueous solution for dilution, and added dichloromethane for extraction. Two times, 200ml each time, the dichloromethane phases were combined, concentrated under reduced pressure to evaporate the dichloromethane, then 300ml of acetone was added, the temperature was raised to dissolve the clear, cooled to 0-10°C for crystallization to obtain 1-(4-methoxybenzyl )-3,4,5,6,7,8-hexahydroisoquinoline hydrochloride 99.8 g, content 99.3%, arylate impurity 1-(4-methoxybenzyl)-5,6, 7,8-tetrahydroisoquinoline content 0.3%.
Embodiment 2
[0016] Add 150 milliliters of xylene, 110 grams of N-(2-(cyclohexenylethyl)-2-(4-methoxyphenyl)acetamide to the three-necked flask. Be warmed up to 85-90 ° C and controlled at this temperature Add 89.6g of phosphorus oxychloride dropwise, keep the temperature at 85-90°C for 3 hours, and cool to room temperature. Concentrate under reduced pressure below 70°C, add 80ml of 80% methanol aqueous solution and keep warm for 30min, add 320ml of aqueous solution for dilution, add chloroform for extraction twice , each time with 200ml, combine the dichloromethane phases, concentrate under reduced pressure to evaporate chloroform, then add 300ml of acetone, heat up the solution, cool to 0-10°C for crystallization to obtain 1-(4-methoxybenzyl)-3, 4,5,6,7,8-Hexahydroisoquinoline hydrochloride 95.8 g, content 98.7%, arylate impurity 1-(4-methoxybenzyl)-5,6,7,8- The content of tetrahydroisoquinoline is 0.8%.
Embodiment 3
[0018] Add 150 milliliters of xylene, 110 grams of N-(2-(cyclohexenylethyl)-2-(4-methoxyphenyl)acetamide to the three-necked flask. Be warmed up to 85-90 ° C and controlled at this temperature 89.6g of phosphorus oxychloride was added dropwise, kept at 85 to 90°C for 3 hours, and cooled to room temperature. Concentrated under reduced pressure below 70°C, added 80ml of 80% methanol aqueous solution for 30min, then added 320ml of aqueous solution for dilution, and added dichloromethane for extraction. Two times, 200ml each time, the dichloromethane phases were combined, concentrated under reduced pressure to evaporate chloroform, then 400ml of ethyl acetate was added, the temperature was warmed to dissolve the clear, cooled to 0-10°C for crystallization to obtain 1-(4-methoxybenzyl )-3,4,5,6,7,8-hexahydroisoquinoline hydrochloride 97.8 g, content 98.9%, arylate impurity 1-(4-methoxybenzyl)-5,6, 7,8-tetrahydroisoquinoline content 0.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com