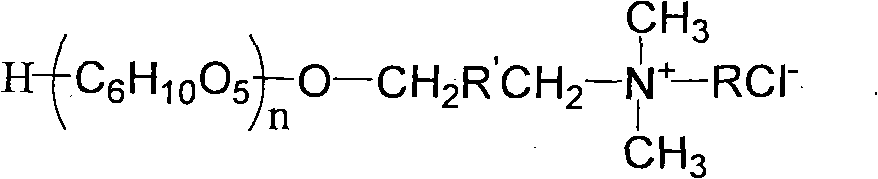Preparation method of glucoside cationic surfactant
A surfactant and cation technology, which is applied in the field of preparation of alkyl glycoside derivatives, can solve the problems of lack of detailed analysis of reaction degree, low final yield, long reaction time, etc., achieving good separation effect and shortening reaction time. time, the effect of improving conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Into a 250 mL four-necked flask equipped with a condenser, a thermometer and a stirrer, 36.0 g of anhydrous glucose, 44.2 g of 3-chloro-1,2-propanediol and 0.3 g of p-toluenesulfonic acid were added. React at a pressure of 200 mmHg and a temperature of 90° C. for 60 minutes to obtain 76.6 g of a light yellow transparent viscous liquid, wherein the conversion rate of glucose is 99.6%. Neutralize to PH≈7 with saturated NaOH aqueous solution. Then the neutralized solution was added to a molecular still, the temperature was controlled to be 100° C., and the pressure was 1 mba to remove excess 3-chloro-1,2-propanediol. 3-Chloro-2-hydroxypropyl glucoside was obtained as a solid. Dissolve the chloroglycoside in water to prepare a 50% aqueous solution.
[0028] In a 250mL four-neck flask equipped with a condenser, a thermometer and a stirrer, add 65g of the above chloroglucoside solution, stir and heat. When the reaction temperature was 50° C., a mixed solution of 15.5 g of ...
Embodiment 2
[0030] Into a 250 mL four-necked flask equipped with a condenser, a thermometer, and a stirrer, 36.0 g of anhydrous glucose, 66.3 g of 3-chloro-1,2-propanediol, and 0.3 g of sulfosuccinic acid were added. React at a pressure of 200 mmHg and a temperature of 110° C. for 50 minutes to obtain 96.2 g of a light yellow transparent viscous liquid, wherein the conversion rate of glucose is 99.5%. Neutralize with saturated KOH aqueous solution to pH ≈ 8, then add the neutralized solution into a molecular still, control the temperature at 160°C, and pressure 3mba to remove excess 3-chloro-1,2-propanediol to obtain 3 - Chlorinated 2-hydroxypropyl glucoside. Dissolve the chloroglycoside in water to prepare a 40% aqueous solution.
[0031] In a 250mL four-neck flask equipped with a condenser, a thermometer and a stirrer, add 81g of the above chloroglycoside aqueous solution, stir and heat. When the reaction temperature was 50° C., a mixed solution of 11 g of dodecyldimethyl tertiary ami...
Embodiment 3
[0033] Into a 250 mL four-necked flask equipped with a condenser, a thermometer, and a stirrer, 36.0 g of anhydrous glucose, 56.7 g of 3-chloropropanol, and 0.20 g of p-toluenesulfonic acid were added. React at a pressure of 200 mmHg and a temperature of 90° C. for 120 minutes to obtain 86.4 g of light yellow transparent viscous liquid, wherein the conversion rate of glucose is 99.7%. Neutralize to pH ≈ 7 with saturated NaOH aqueous solution, then add the neutralized solution into a molecular still, control the temperature at 150 ° C, and the pressure at 2.8 mba to remove excess 3-chloropropanol to obtain solid 3-chloropropanol Propyl glucoside. Dissolve the solid 3-chloropropyl glucoside in water to prepare a 50% aqueous solution.
[0034] In a 250mL four-neck flask equipped with a condenser, a thermometer and a stirrer, add 65g of the above-mentioned 3-chloropropyl glucoside aqueous solution, stir and heat. When the reaction temperature was 50° C., a mixed solution of 8.8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com