Method for preparing dipeptidyl peptidase-IV inhibitor
A compound, the technology of camphorsulfonic acid, applied in the field of compound preparation, can solve the problems of high product cost and expensive reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
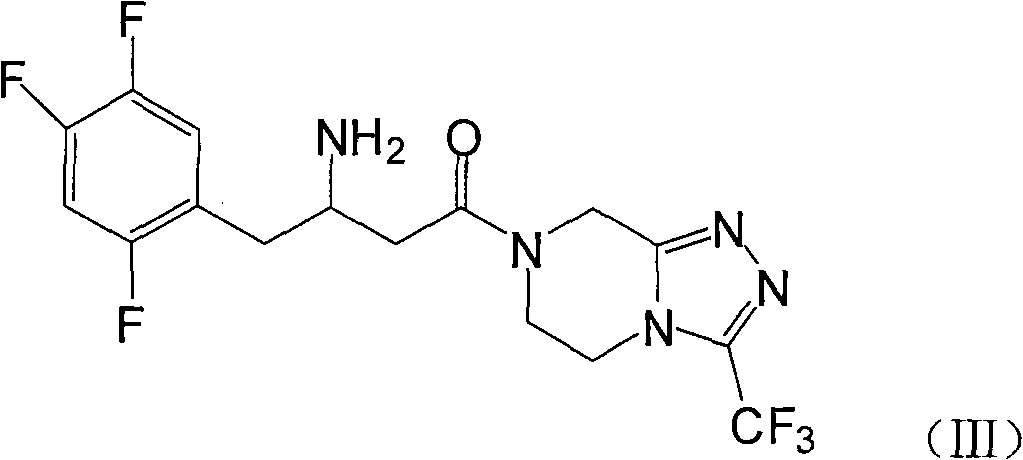
[0028] Example 1: 7-[3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-trifluoromethane Preparation of base-1,2,4-triazolo[4,3-a]pyrazine (III)
[0029] Add 10g of compound (II) and 110ml of methanol into the reaction kettle, heat up to about 40°C and stir to dissolve, add 2g of palladium carbon, pressurize and roughly estimate the hydrogen absorption according to the theoretical amount, about 6-8 hours, after the reaction is completed, filter out the palladium Carbon, 200ml isopropanol was added to the filtrate, stirred for 1 hour, and compound III was obtained by filtration.
Embodiment 2
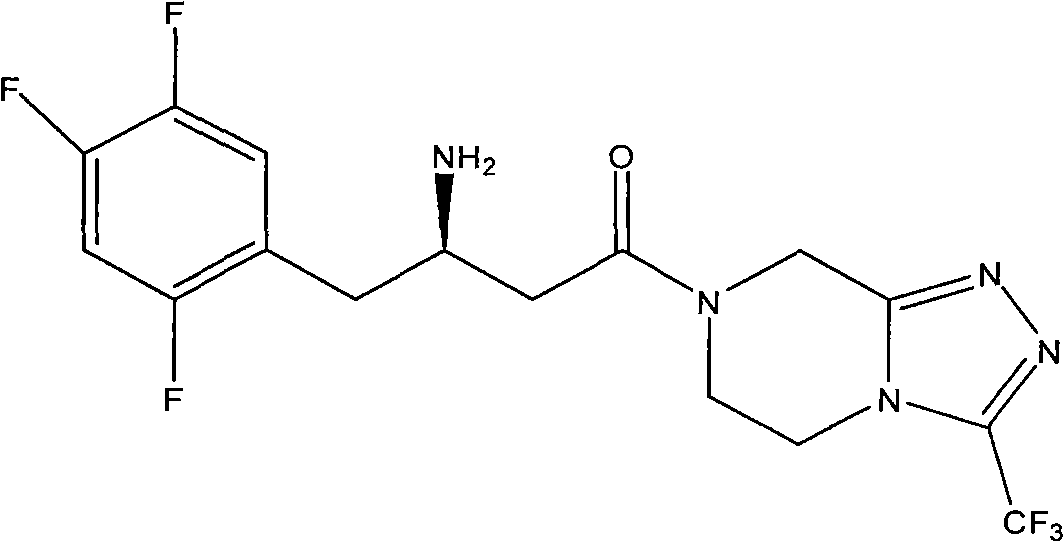
[0030] Example 2: 7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3 -Preparation of trifluoromethyl-1,2,4-triazolo[4,3-a]pyrazine camphorsulfonate
[0031] Dissolve 10 g of compound III in 100 ml of toluene, add 6.2 g of D-camphorsulfonic acid monohydrate, heat and stir overnight. Cool, filter, and recrystallize the solid from toluene. Camphorsulfonate is obtained.
Embodiment 3
[0032] Example 3: 7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3 - Preparation of trifluoromethyl-1,2,4-triazolo[4,3-a]pyrazine tartrate
[0033] Dissolve 10 g of compound III in 100 ml of toluene, add 3.7 g of (-)-tartaric acid, and react at room temperature for 48 hours. A white solid precipitated out of the system, was filtered, and the solid was recrystallized from toluene. Get tartrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com