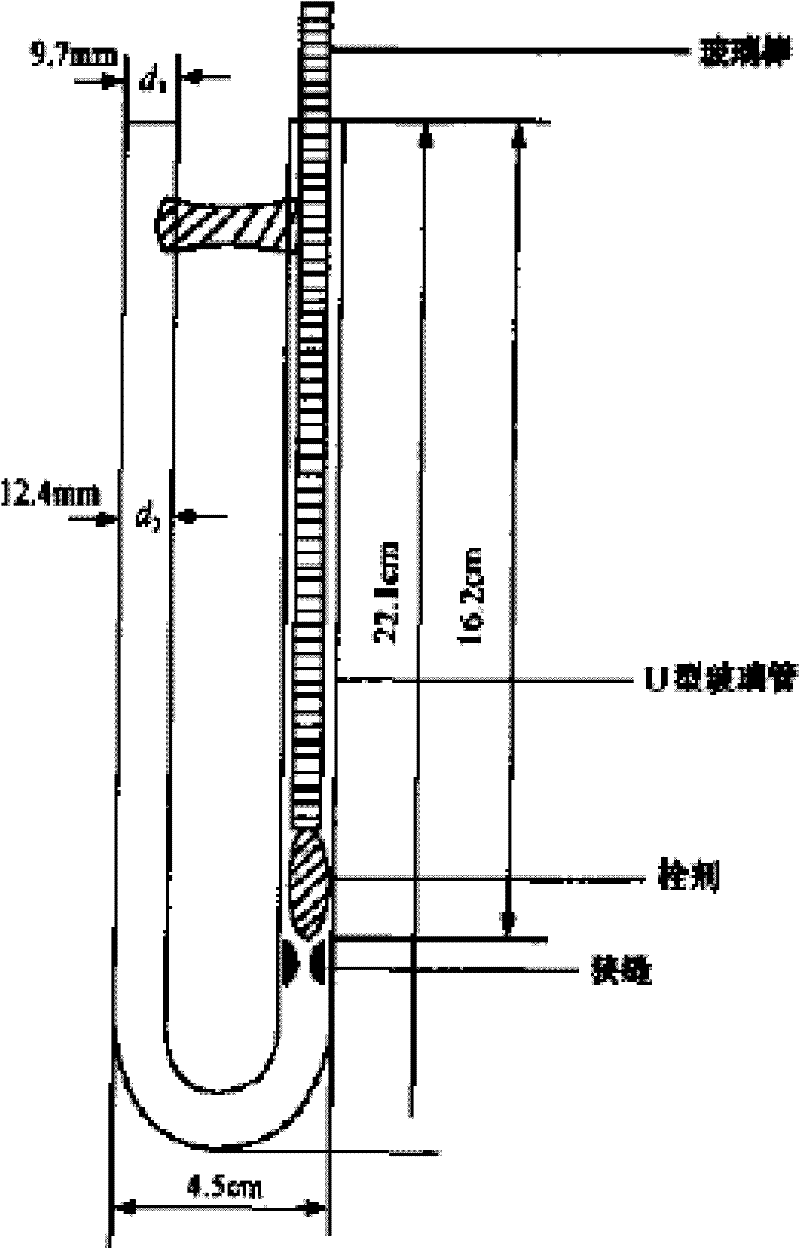
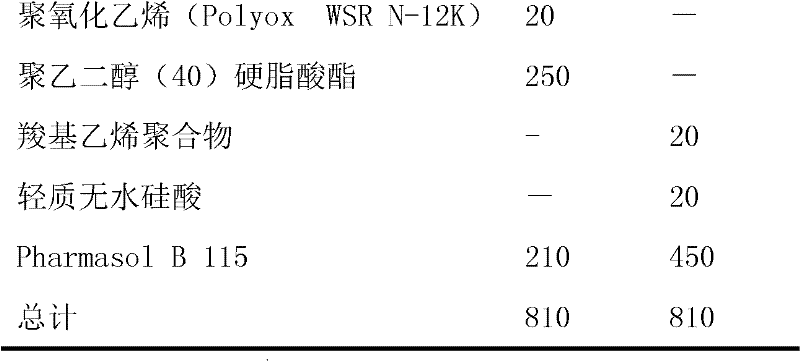
Suppository composition
A composition and suppository technology, applied in the field of suppository compositions with improved performance, can solve the problems of rising melting point, poisoning gel, difficult to mix evenly, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The above-mentioned suppository composition will be described in detail below.
[0024] The aliphatic suppository base used in the present invention can be fatty acid glycerides, such as fatty acid monoglycerides, fatty acid diglycerides, fatty acid triglycerides and mixtures thereof, where the fatty acids are usually C10-C18. Pure or mixed fatty acids, preferably pure or mixed fatty acids with C14-C18 carbon atoms, such as vegetable fatty acids obtained from coconut oil, olive oil. The melting point of these fatty acid glycerides is usually not lower than 25°C, preferably not lower than 37°C, but preferably not higher than 45°C, more preferably not higher than 42°C. Examples of fatty acid glycerides useful in the present invention are (manufactured by Gattefosse Co., Ltd), (manufactured by Dynamic Nobel Chemicals Co.Ltd), Pharmasol (manufactured by Nippon Oils and Fats), (manufactured by Aarhus), or (manufactured by Karlshamns) (manufactured by Cognis), (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com