Preparation method for B cell CD 22 extracellular inhibitive peptide fragment B2285 vaccines
An inhibitory, B cell technology, applied in the field of preparation of B cell CD22 extracellular inhibitory peptide segment B2285 vaccine, can solve the problems of short action time, frequent administration, and difficulty in promotion, and achieves low production cost and high safety. , the effect of less toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
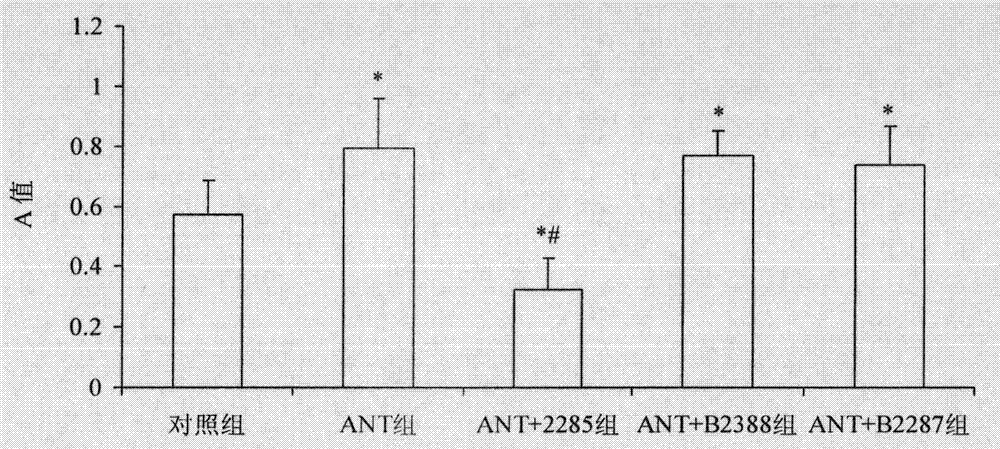
[0048] Example 1 Screening of B cell mCD22 extracellular inhibitory peptide B2285 and its therapeutic observation on ANT-induced immune dilated cardiomyopathy:
[0049] Screening of B cell mCD22 extracellular inhibitory peptides of the present invention and their effects on ANT-induced immune dilated cardiomyopathy mice
[0050] 1. Materials and methods
[0051] 1.1 Experimental animals
[0052] Select 40 inbred BALB / c mice, male, 6 weeks old, weighing about 16 grams, purchased from the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology. All experimental animals were kept in the clean animal room of the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology.
[0053] 1.2 Preparation of B cell mCD22 extracellular peptide antigen vaccine
[0054] 1.2.1 Design of B cell mCD22 extracellular peptide
[0055] The amino acid sequence of the specific molecule CD22 on the surface of mouse ...
Embodiment 2
[0123] Example 2 The therapeutic observation of B cell mCD22 extracellular inhibitory peptide B2285 on spontaneous MRL / lpr lupus mice mainly mediated by B cells:
[0124] In vitro experiment of the present invention
[0125] 1. Materials and Methods
[0126] 1.1 Experimental animals
[0127] Select 6 SPF-grade MRL / lpr female lupus mice, 11-12 weeks old, weighing 36-40g, provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences; select SPF-grade female C57BL / 6 mice that are isotype-controlled with MRL / lpr lupus mice Ten rats, 10 weeks old, weighing 30-35g, were provided by the Experimental Animal Center of Wuhan University School of Medicine. All experimental animals were kept in the clean animal room of the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology.
[0128] 1.2 Preparation of B2285 antigen vaccine
[0129] The method is the same as before.
[0130] 1.3 Preparation and extraction of ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com