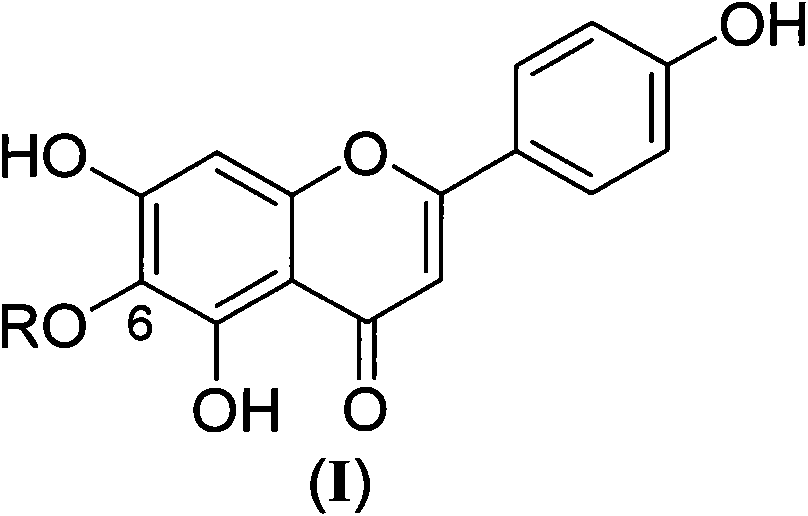
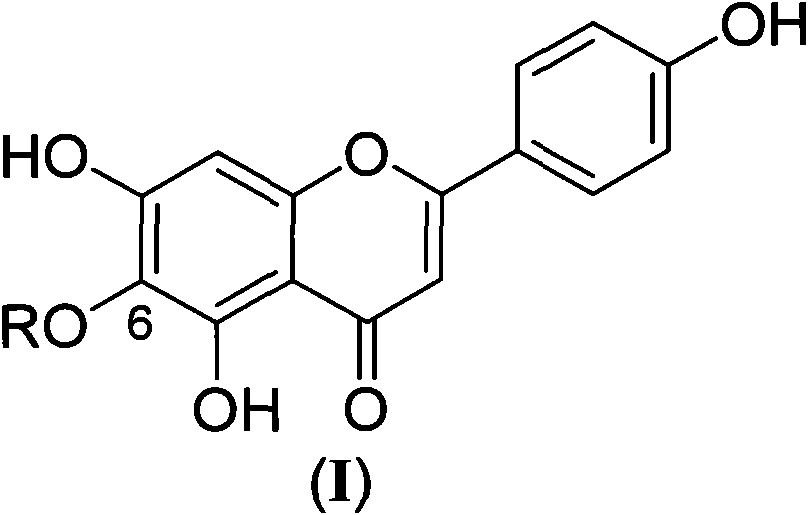
Scutellarein derivative as well as preparation method and application thereof
A technology of scutellarin aglycone and scutellarin aglycon, which is applied in the field of medicinal chemistry research, can solve the problems of low adverse reactions and high bioavailability, and achieve the effects of low adverse reactions, high bioavailability, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
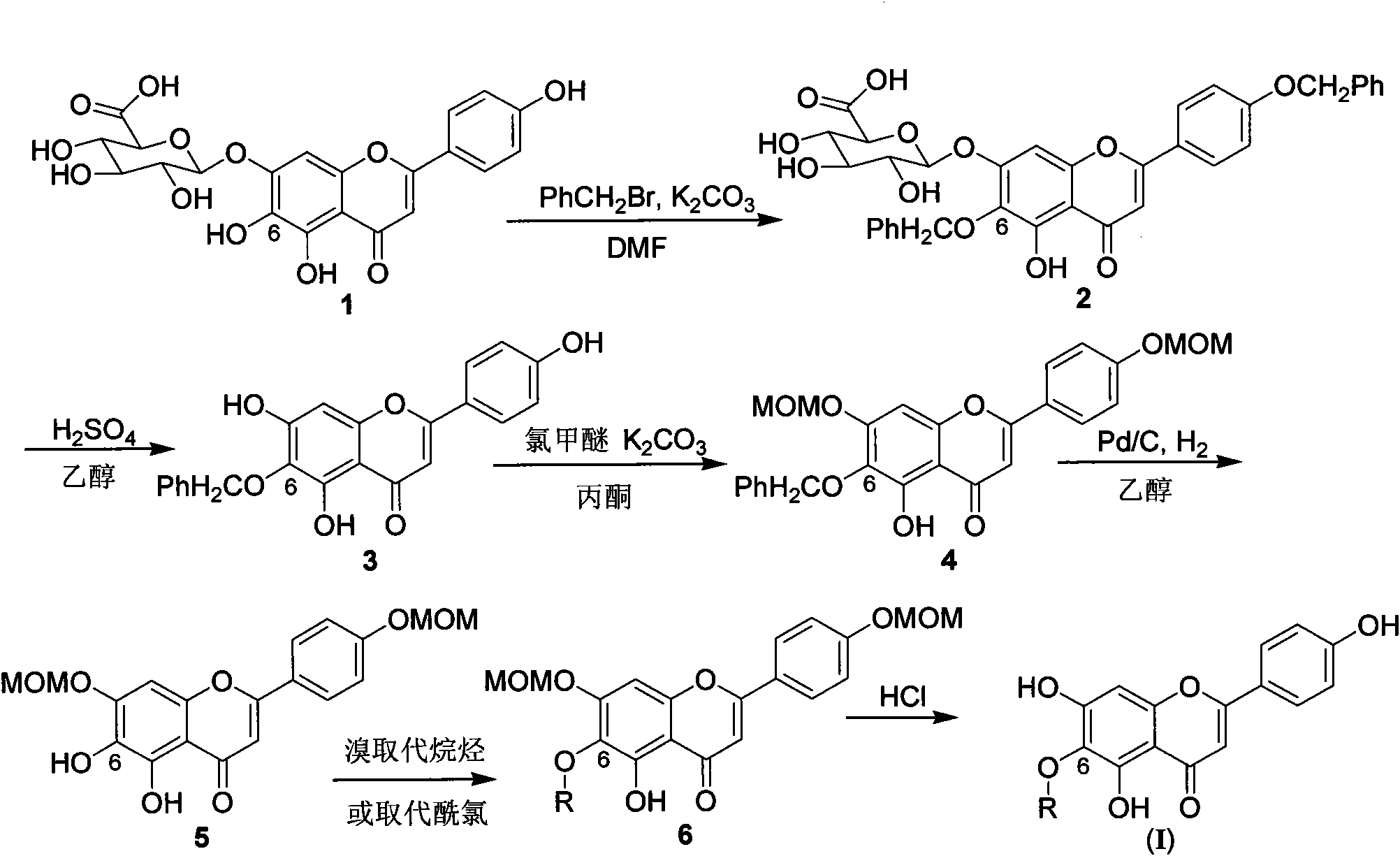
[0043] Example 1 4', the preparation of 6-dibenzyloxy-5-hydroxyl-7-glucuronic acid oxyflavone (2)
[0044] Add scutellarin 2.4g, DMF, K 2 CO 3 (2.5equiv), under the protection of inert gas nitrogen, add benzyl bromide (2.3equiv), react at 25°C for 8 hours, after the reaction, add ice water, control PH≤6, precipitate solid, suction filter, dry to obtain yellow solid 2.7 grams. Yield 81%.
[0045] The obtained compound spectral data is:
[0046] MS (TOF, m / z): 627.01 [M-H] - .
[0047] 1 HNMR (DMSO-d 6 , 300MHz): 12.97 (5-OH), 7.91 (2H, d, j=8.58, 2', 6'-ArH), 6.92 (2H, d, j=8.58, 3', 5'-ArH), 6.74 (1H, s, 3-H), 6.57 (1H, s, 8-H), 7.20 (10H, Ar-H), 5.22 (2H, s, H-CHAr), 5.16 (2H, s, H-CHAr ), 5.51 (1H, d, J=12, 1”-H).
Embodiment 2
[0048] Example 2 4', 5, the preparation of 7-trihydroxy-6-benzyloxyflavone (3)
[0049] Take 23.2 g of the compound prepared in Example 1, add it to 1 mol / L sulfuric acid ethanol solution, and react at 80°C for 12 hours under the protection of an inert gas. After the reaction, add ice water, control the pH at 5-6, and precipitate yellow The solid was filtered with suction, and the obtained crude product was purified by column chromatography to obtain 0.9 g of a yellow solid (eluent: dichloromethane: methanol). Yield 48%.
[0050] The obtained compound spectral data is:
[0051] MS (TOF, m / z): 375.01 [M-H] - .
[0052] 1 HNMR (DMSO-d 6 , 300MHz): 12.97 (5-OH), 10.44 (1H, s, 7-OH), 10.30 (1H, s, 4'-OH), 7.91 (2H, d, j=8.58, 2', 6'- ArH), 6.92 (2H, d, j=8.58, 3', 5'-ArH), 6.74 (1H, s, 3-H), 6.57 (1H, s, 8-H), 7.20 (5H, Ar- H), 5.18 (2H, s, 1”-H).
Embodiment 3
[0053] Example 3 4', the preparation of 7-dimethoxymethylene oxygen-5-hydroxyl-6-benzyloxyflavone (4)
[0054] 1.65g of K 2 CO 3 , 1.9 g of intermediate 3 were put into 30 ml of acetone in turn, stirred at room temperature for 30 minutes, then added MOMCl (1.5 equiv), heated to room temperature at 70° C. and stirred for 12 hours. After the reaction, add an appropriate amount of ice water to control the pH to 4-7, filter with suction, and wash the filter cake with a small amount of ice water. The obtained crude product was recrystallized to obtain 1.7 g of a yellow solid (solvent: ether / ethanol). Yield: 72.6%
[0055] The obtained compound spectral data is:
[0056] MS (TOF, m / z): 463.15 [M-H] - .
[0057] 1 HNMR (DMSO-d 6 , 300MHz): 12.97 (5-OH), 7.91 (2H, d, j=8.58, 2', 6'-ArH), 6.92 (2H, d, j=8.58, 3', 5'-ArH), 6.74 (1H, s, 3-H), 6.57 (1H, s, 8-H), 7.20 (5H, m, Ar-H), 5.22 (2H, s, 1”-H), 5.16 (2H, s, -OCH 2 O-), 5.23 (2H, s, -OCH 2 O-), 3.21 (3H, s, -OCH 3 ), 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com