Chinese medicinal injectable powder and quality control method thereof
A technology of powder injection and traditional Chinese medicine, which is applied in the fields of medical formula, powder delivery, measuring device, etc. It can solve the problems that affect the reliability and stability of the measurement results, the detection time of multiple steps, and the large amount of organic solvents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] 1 part of red ginseng, 3 parts of Ophiopogon japonicus, 1.5 parts of schisandra
[0134] Take the above-mentioned three-flavored medicinal materials in the proportion by weight, red ginseng is extracted three times with ethanol reflux, the first and second times are 3 hours respectively, and the third time is 2 hours, the extracts are combined, the ethanol is recovered under reduced pressure until there is no alcohol smell, and water is added to about 500ml, place in cold place, separate and remove the upper layer oil, filter the water layer, concentrate under reduced pressure to a relative density of 1.15-1.20 (50°C), dry under reduced pressure at 70°C to obtain red ginseng extract for injection; Ophiopogon japonicus and Schisandra chinensis add water respectively Decoct three times, 1 hour for the first time, 45 minutes and 30 minutes for the second and third times respectively, combine the extracts, concentrate under reduced pressure to relative densities of 1.15-1.20...
Embodiment 2
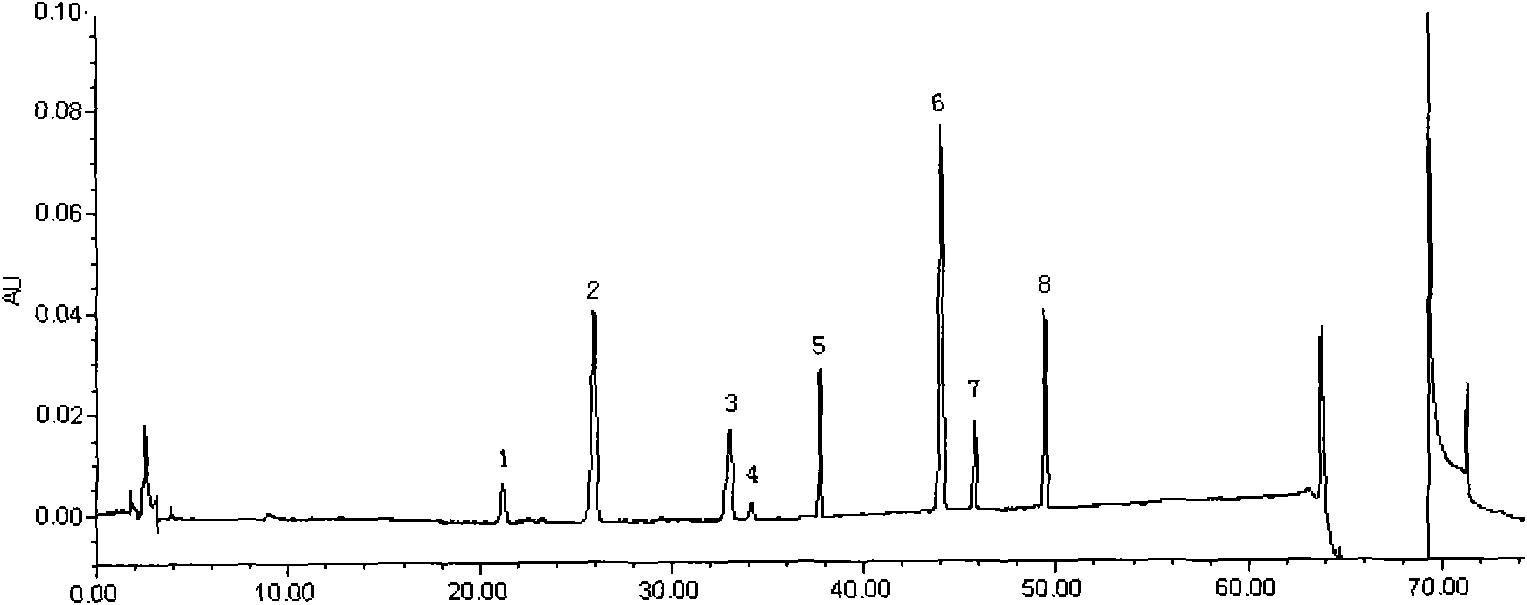
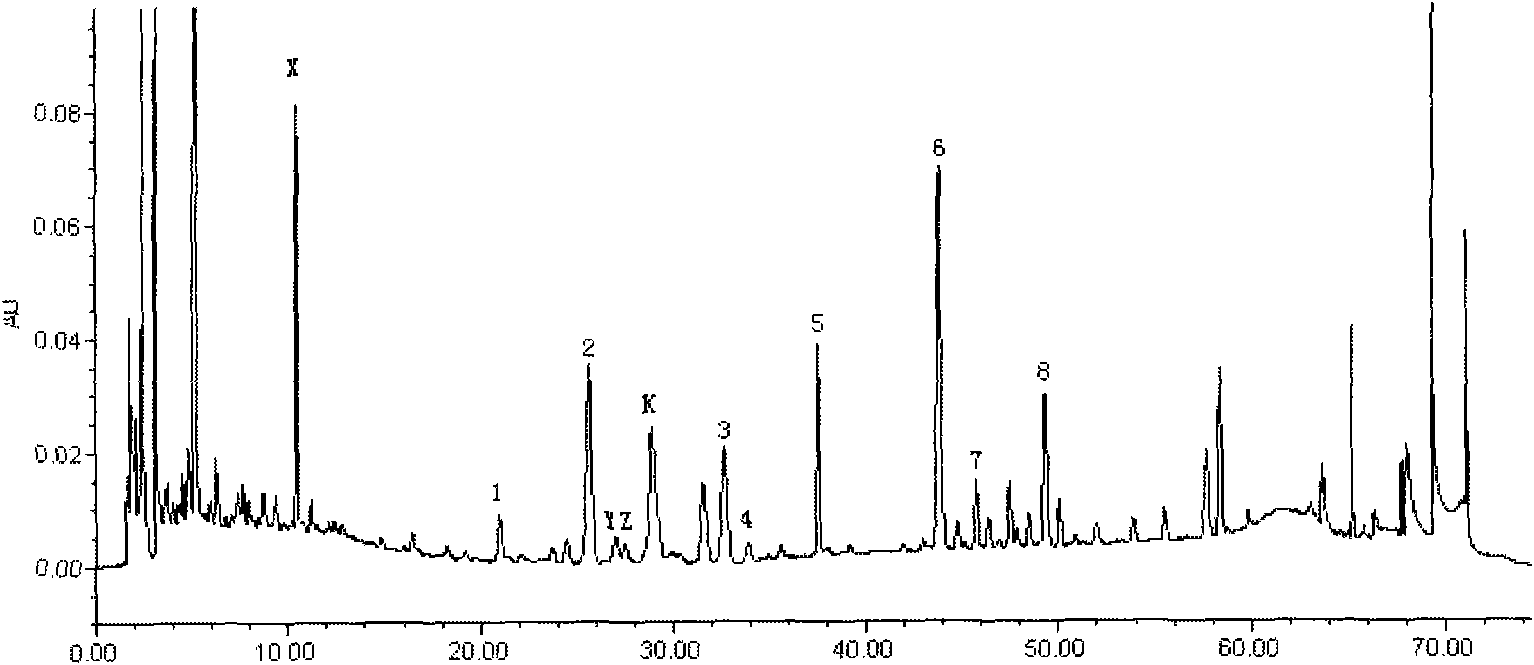
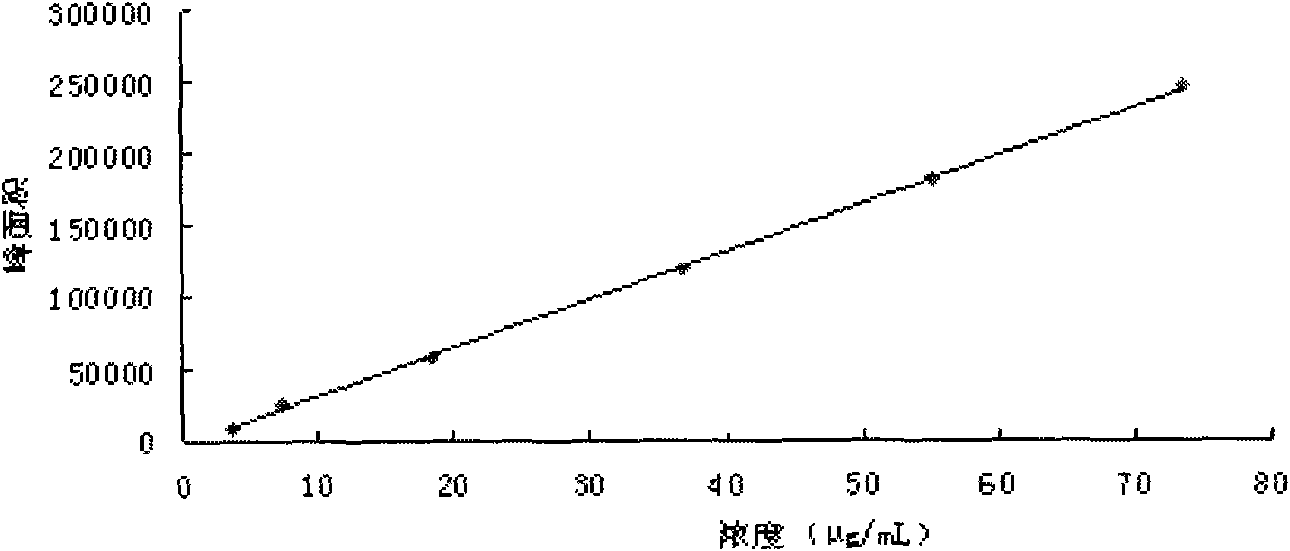
[0136] Embodiment 2 Assay method
[0137] 1 Instruments and materials
[0138]1.1 Instruments Waters2695 high-performance liquid chromatography unit, equipped with Waters2996 diode array detector (WATERS, USA); XS105 1 / 100,000 electronic analytical balance (Switzerland METTLER) Milli-Q ultrapure water treatment system.
[0139] 1.2 Materials Ginsenoside Rf, Rb 1 , Rb 2 , Rb 3 , Rd, Schisandrin A, F 2 Reference substance (for content determination, provided by China Institute for the Control of Pharmaceutical and Biological Products); Yiqi Fumai (freeze-dried) sample for injection is Example 1; acetonitrile is chromatographically pure; phosphoric acid is analytically pure; water is high-purity water.
[0140] 2 Experimental methods
[0141] 2.1 Chromatographic conditions Chromatographic column: Waters Symmetry C 18 Column (4.6mm × 250mm, 5 mm), mobile phase: 0.05% phosphoric acid-acetonitrile composition, gradient elution according to the gradient ratio in Table 1; detect...
Embodiment 3
[0153] Get 0.5 part of Schisandra chinensis, 0.5 part of red ginseng, and 6 parts of Ophiopogon japonicus prepared according to the preparation method of Example 1 to prepare the preparation, and measure 0.35 mg / g of ginsenoside Rf content and 0.35 mg / g of ginsenoside Rb according to the content determination method of Example 2. 1 Content 1.4mg / g, Ginsenoside Rb 2 Content 0.8mg / g, Ginsenoside Rb 3 Content 0.12mg / g, ginsenoside Rd content 1.0mg / g, ginsenoside Rg3 content 0.6mg / g, ginsenoside F 2 content of 0.3mg / g and Schisandrin A content of 0.1mg / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com