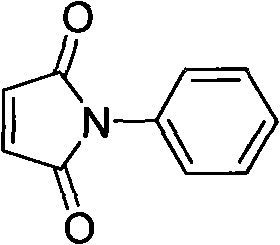
Method for synthesizing N-benzyl maleimide from immobilized supported acid catalyst
A maleimide, catalyst-loaded technology, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of large pollution in the production process, easy wear and tear of catalysts, and many production processes, and reduce waste. Acid content, reduction of water washing, and simplification of the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) with 10% H 3 PO 4 The equal volume of the solution was impregnated with silica gel for 6 hours, then vacuum-dried and dried at high temperature to obtain a loaded catalyst; then 4 grams of loaded catalyst was packed into a stainless steel mesh and fixed on a stirrer;
[0019] 2) 9.3 grams of aniline and 8.8 grams of maleic anhydride were added to 70 milliliters of xylene for acylation, stirred, heated at a temperature of 100° C., and reacted for 0.5 hours to obtain an N-phenylmaleamic acid solution;
[0020] 3) Add N-phenylmaleamic acid solution, 0.2 g of hydroquinone and a supported catalyst into a dehydration reactor for dehydration and ring closure, the reaction temperature is 160° C., the reaction time is 1 hour, and the solvent is removed under reduced pressure to obtain Crude N-phenylmaleimide;
[0021] 4) The obtained crude N-phenylmaleimide was recrystallized in 200 ml of cyclohexane to obtain N-phenylmaleimide with a yield of 92%.
Embodiment 2
[0023] 1) with 10% H 3 PO 4 The equal volume of the solution was impregnated with silica gel for 6 hours, then vacuum-dried and dried at high temperature to obtain a loaded catalyst; then 2 grams of loaded catalyst was packed into a stainless steel mesh and fixed on a stirrer;
[0024] 2) 9.3 grams of aniline and 9.2 grams of maleic anhydride were added to 70 milliliters of xylene for acylation, stirred, heated at a temperature of 90° C., and reacted for 1 hour to obtain an N-phenylmaleamic acid solution;
[0025] 3) Add N-phenylmaleamic acid solution, 0.1 gram of hydroquinone, 0.1 gram of cuprous chloride and a supported catalyst into a dehydration reactor for dehydration and ring closure, the reaction temperature is 150°C, and the reaction time is 2 hours , remove the solvent under reduced pressure to obtain the crude product of N-phenylmaleimide;
[0026] 4) The obtained crude N-phenylmaleimide was recrystallized in 100 ml of ethanol to obtain N-phenylmaleimide with a yie...
Embodiment 3
[0028] 1) with 10% H 3 PO 4 The equal volume of the solution was impregnated with silica gel for 6 hours, then vacuum-dried and dried at high temperature to obtain a loaded catalyst; then 4 grams of loaded catalyst was packed into a stainless steel mesh and fixed on a stirrer;
[0029] 2) 9.3 grams of aniline and 9.8 grams of maleic anhydride were added to 70 milliliters of toluene for acylation, stirred, heated at a temperature of 80°C, and reacted for 2 hours to obtain an N-phenylmaleamic acid solution;
[0030] 3) Add N-phenylmaleamic acid solution, 0.3 g of cuprous chloride and a supported catalyst into a dehydration reactor for dehydration and ring closure, the reaction temperature is 150 ° C, the reaction time is 2 hours, and the solvent is removed under reduced pressure to obtain Crude N-phenylmaleimide;
[0031] 4) Recrystallize the crude N-phenylmaleimide obtained in 200 ml of cyclohexane to obtain N-phenylmaleimide with a yield of 87%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com