Method for detecting deuterium content in deuterium depleted water
A technology of ultra-light water and deuterium content, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of high stability requirements of instruments and huge investment in instruments and equipment, and achieve compact and reasonable design, low equipment investment, and low system cost. effect of error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Instruments: GC8800H gas chromatograph, thermal conductivity detector, HL-3000 chromatography workstation.
[0031] 2. Chromatographic conditions: high-purity hydrogen as the carrier gas, the purity of which is above 99.99%, the carrier gas flow rate is 20ml / min, the stainless steel chromatographic column, Φ3×2m, filled with 5A molecular sieve, the column temperature is 40℃, the detector is 50℃, The sample temperature is room temperature, and the bridge current is 100mA.
[0032] 3. Preparation of standard samples:
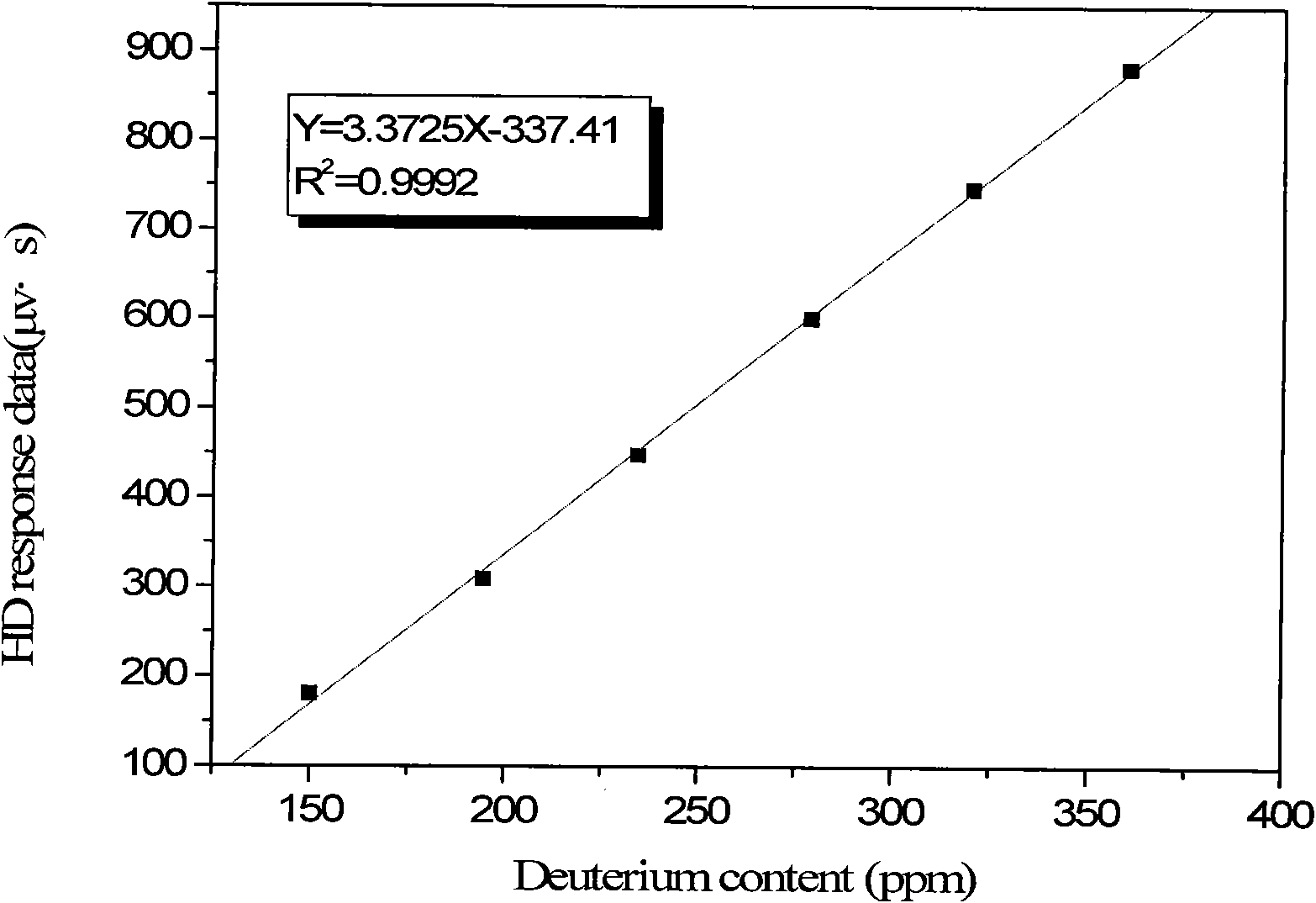
[0033] Several standard samples with different deuterium content were accurately prepared from 99.9% heavy water and Shanghai tap water by a stepwise dilution method. The atomic percentages were 194.4ppm, 234.4ppm, 278.9ppm, 320.3ppm, 360.1ppm.
[0034] 4. Drawing of standard curve:
[0035] The above-mentioned standard samples were reduced with magnesium bars under vacuum. First evacuate the system, then heat the reaction tube with magnesium bars to 500°C, inje...
Embodiment 2
[0045] 1. Instruments: GC8800H gas chromatograph, thermal conductivity detector, HL-3000 chromatography workstation.
[0046] 2. Chromatographic conditions: high-purity hydrogen as the carrier gas, its purity is above 99.99%, the carrier gas flow rate is 30ml / min, stainless steel chromatographic column, Φ3×2m, filled with 5A molecular sieve, column temperature 60℃, detector 70℃, inlet The sample temperature is room temperature, and the bridge current is 110mA.
[0047] 3. Preparation of standard samples:
[0048] Several standard samples with different deuterium content were accurately prepared from 99.9% heavy water and Shanghai tap water by a stepwise dilution method. The atomic percentages were 194.4ppm, 234.4ppm, 278.9ppm, 320.3ppm, 360.1ppm.
[0049] 4. Drawing of standard curve:
[0050] The above-mentioned standard samples were reduced with zinc under vacuum. First evacuate the system, then heat the reaction tube filled with zinc chips to 460℃, use a syringe to inject 0.1ml of ...
Embodiment 3
[0062] 1. Instruments: GC8800H gas chromatograph, thermal conductivity detector, HL-3000 chromatography workstation.
[0063] 2. Chromatographic conditions: high-purity hydrogen as the carrier gas, its purity is above 99.99%, the carrier gas flow rate is 40ml / min, stainless steel chromatographic column, Φ3mm×3m, filled with 5A molecular sieve, column temperature 40℃, detector 70℃, enter The sample temperature is room temperature, and the bridge current is 80mA.
[0064] 3. Preparation of standard samples:
[0065] Several standard samples with different deuterium content were accurately prepared from 99.9% heavy water and Shanghai tap water by a stepwise dilution method. The atomic percentages were 194.4ppm, 234.4ppm, 278.9ppm, 320.3ppm, 360.1ppm.
[0066] 4. Drawing of standard curve:
[0067] The above-mentioned standard samples were reduced with zinc under vacuum. First evacuate the system, then heat the reaction tube filled with zinc chips to 500°C, inject 0.1ml of water sample in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com