Preparation of 6-oxa-8alpha-steroid estrogen analogues - a new group of unnatural estrogens and their use in medicine
A technology for steroidal estrogens and analogs, applied in the field of novel 6-oxa-8α-steroidal estrogen analogs, can solve the problems of increasing the risk of uterine cancer and endometrial cancer, and use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
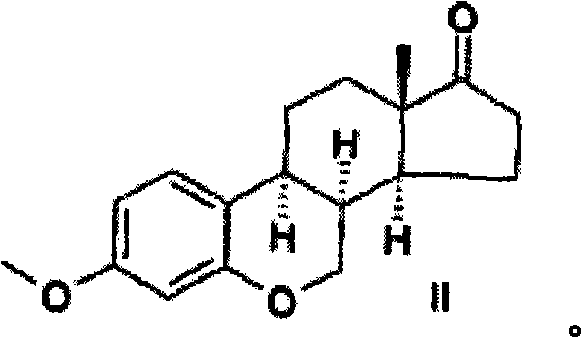
[0055] Example 1: 6-oxa-8α-estrone methyl ether (H)
[0056]
[0057]To 3-methoxy-6-oxaestra-1,3,5(10), 8,14-penten-17-one (3-methoxy-6-oxaestra-1,3,5(10) , 8,14-pentaen-17-one) I (1 g) in THF solution (50 ml) was added 10% Pd / C (300 mg) to synthesize compound II. The hydrogenation process was monitored by UV measurements. The reaction was stopped when the aromatic characteristic wavelength disappeared. The catalyst was then filtered and washed with THF (50ml). The organic layer was collected and the solvent was removed under vacuum. Make the residue from CHCl 3 -MeOH mixture crystallized out.
[0058] The yield of the target sterol II was 64% (0.65 g), and the melting point was 149-150°C.
[0059] NMR 1 H in CDCl 3 The results shown in (δ, ppm) are as follows: 0.93s (3H, C 13 -CH 3 ), 1.43 (1H, C 12α -H), 1.68 (1H, C 11β -H), 1.84 (1H, C 12β -H), 1.90 (2H, C 15α -H and C 15β -H), 1.96 (1H, C 14α -H), 2.00 (1H, C 111α -H), 2.18(1H, C 16α -H), 2.45 (1H, C ...
Embodiment 2
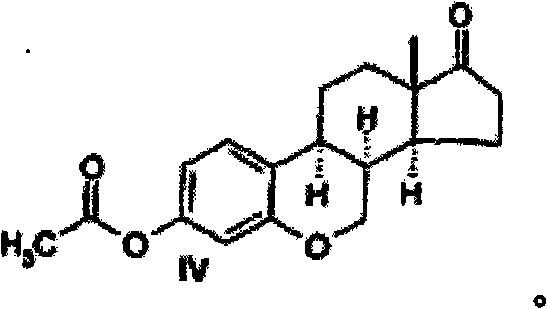
[0062] Example 2: 6-oxa-8α-estrone acetate (IV)
[0063]
[0064] With 3-methoxy-6-oxa-8α-estra-1,3,5(10)-triene-17-one (3-methoxy-6-oxa-8α-estra-1,3,5 (10), 8,14-pentaen-17-one) II (573 mg) was refluxed in HBr and AcOH (20 ml, 3 / 7, v / v) at 70° C. for two hours to synthesize compound IV. The reaction mixture was poured into water; the precipitate was filtered and rinsed with water to neutral pH. The product is then air dried.
[0065] The amount of product obtained after hydrolysis was 440 mg (80.5%). This compound was used in the next stage of synthesis without further purification.
[0066] The above compound was dissolved in 10 ml of pyridine / acetic anhydride mixture (volume ratio 1:9), kept at 100° C. for 2.5 hours, and then left overnight at room temperature. The precipitate was filtered off, washed with hexane and dried under vacuum.
[0067] The final yield of the target steroid IV was 230 mg (37%) with a melting point of 135-138°C.
[0068] NMR 1 H in CDCl 3...
Embodiment 3
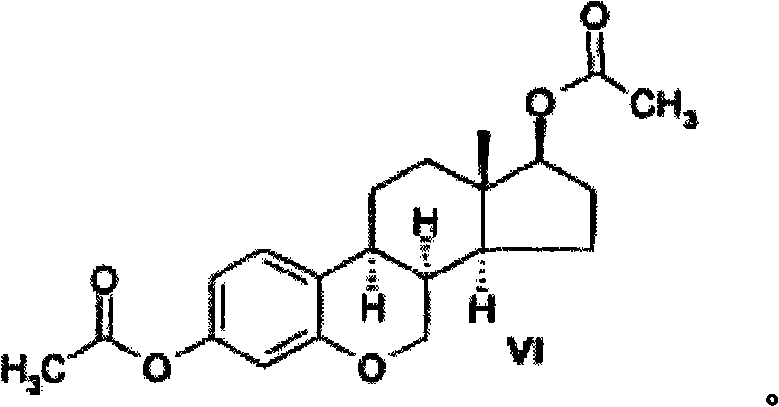
[0071] Example 3: 6-oxa-8α-estradiol diacetate (VI)
[0072]
[0073] To 3,17β-diacetoxy-6-oxestr-1,3,5(10),8,14-pentaene V (1 g) in THF (50 ml) was added 10% of Pd (200mg) to synthesize compound VI. The hydrogenation was carried out under the conditions described in Example 1. The catalyst was filtered off and washed with THF (10ml). The solvent was removed in vacuo and the residue was crystallized from MeOH.
[0074] The yield of the target compound was 0.51 g (50%), and the melting point was 158-160°C.
[0075] NMR 1 H in CDCl 3 The results shown in (δ, ppm) are as follows: 7.07, 1H, d, J=8.0Hz (H-C 1 ); 6.61, 1H, dd, J=2.2Hz, J=8.0Hz (H-C 2 ); 6.54, 1H, d, J=2.2Hz (H-C 4 ); 4.63, 1H, t, J=8.7Hz (H-C 17 ); 4.25-4.15, 1H, m(H α -C 7 ); 4.1-4.0, 1H, m(H β -C 7 ); 2.65-1.3, 11H, m(H-C 8 , H-C 9 , H 2 -C 11 , H 2 -C 12 , H-C 14 , H 2 -C 15 , H 2 -C 16 ); 2.26, 3H, s(H 3 CCOO-C 3 ); 2.05, 3H, s(H 3 CCOO-C 17 ); 0.84, 3H, s(H 3 -C 18 ).
[0076] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com