Fluorine-containing polyimide electro-optical material and preparation method thereof
A technology of hydroxyl fluorinated polyimide and electro-optic materials, applied in optics, nonlinear optics, instruments, etc., can solve the problems of poor solubility of polyimide, achieve excellent comprehensive performance, improve polarization efficiency, and high thermal stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026]
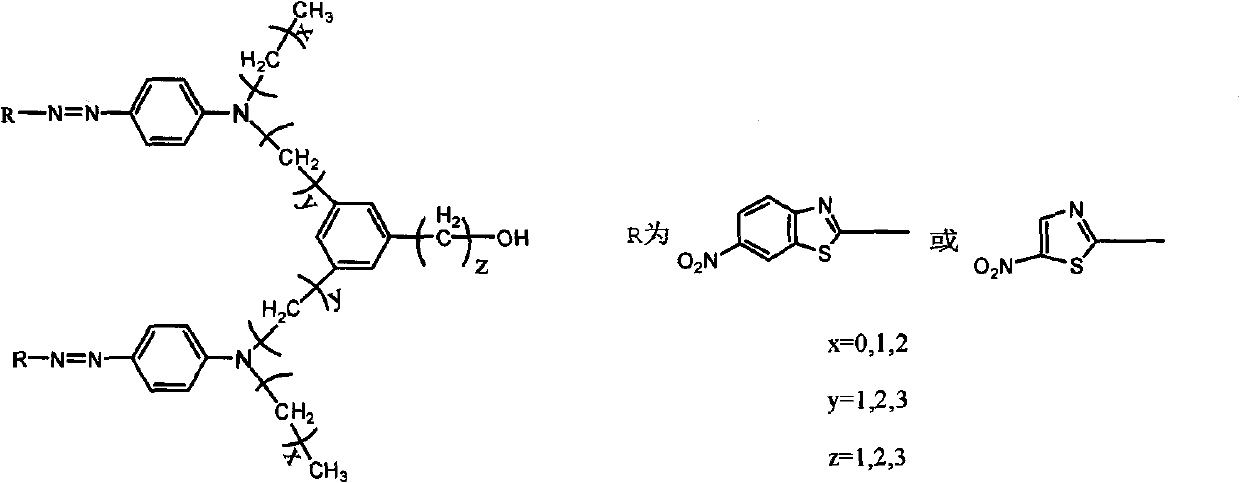
[0027] NLO is a Λ-shaped heterocyclic azo chromogenic molecule containing a thiazole ring, and its structural formula is:
[0028]
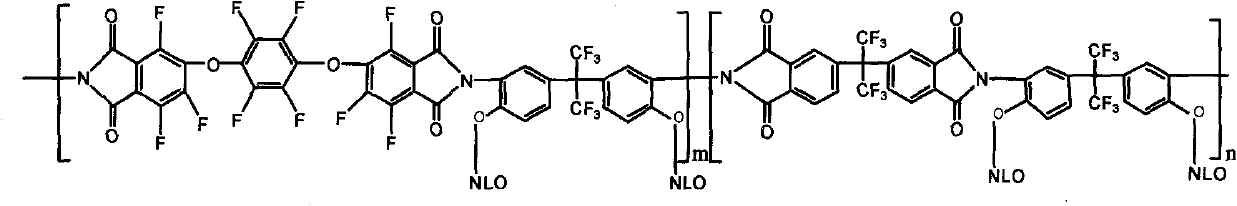
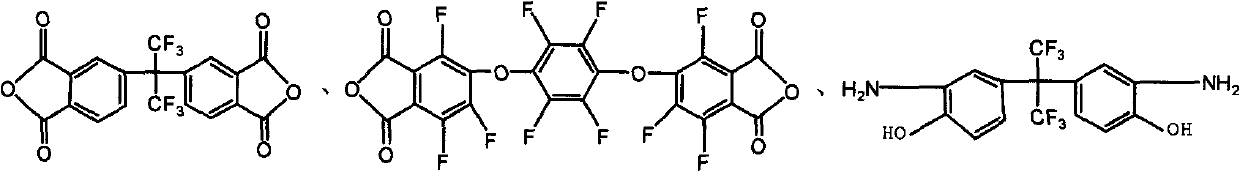
[0029] 4,4'-(hexafluoroisopropyl)-phthalic anhydride (3mmol, dianhydride monomer 6FDA) and 1,4-bis-(3,4-bisformyltrifluorophenoxy)tetrafluorobenzene Dianhydride (3mmol, dianhydride monomer 10FEDA) was dissolved in 8mL N,N-dimethylformamide solution, and added to 5,5'-(hexafluoroisopropyl)-bis-(2 -Aminophenol) (6mmol, diamine monomer 6FHP) in 8mL N,N-dimethylformamide solution, the solution was heated to room temperature and electromagnetically stirred in nitrogen for 24h to generate a polyamic acid solution, and 16mL was added to the flask Dried xylene solution, and then the polyamic acid solution was thermally cyclized at 160°C for 5 hours in a nitrogen atmosphere, while the water generated by the cyclization was taken out by the xylene azeotrope, and the reacted solution was dropped into 50mL of methanol In the mixed solution of H...
example 2
[0031]
[0032] NLO is a Λ-shaped heterocyclic azo chromogenic molecule containing a benzothiazole ring, and its structural formula is:
[0033]
[0034] 4,4'-(hexafluoroisopropyl)-phthalic anhydride (3mmol, dianhydride monomer 6FDA) and 1,4-bis-(3,4-bisformyltrifluorophenoxy)tetrafluorobenzene Dianhydride (3mmol, dianhydride monomer 10FEDA) was dissolved in 8mL N,N-dimethylformamide solution, and added to 5,5'-(hexafluoroisopropyl)-bis-(2- Aminophenol) (6mmol, 6FHP) in 8mL N,N-dimethylformamide solution, the solution was heated to room temperature and magnetically stirred in nitrogen for 24 hours to generate a polyamic acid solution, and 16mL of dry xylene was added to the flask solution, and then this polyamic acid solution was thermally cyclized at 160°C for 5h in a nitrogen environment, and the water produced by the cyclization was taken out by the xylene azeotrope, and the reacted solution was dropped into 50mL of methanol / water (1 : 1, V / V) and 10mL of 2N HCl in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electro-optic coefficient | aaaaa | aaaaa |
| electro-optic coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com