Medical suspended preparation and preparation method thereof
一种药品、贮药的技术,应用在药品悬挂剂及其制备领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
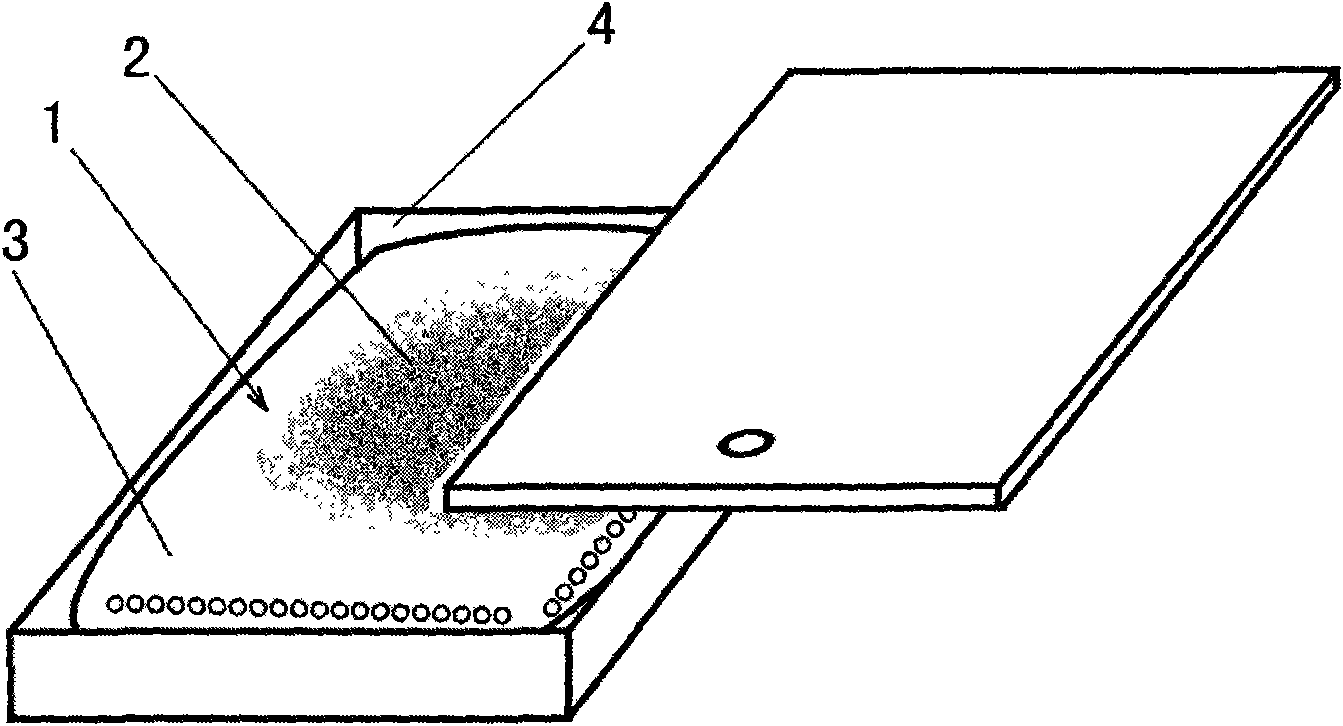
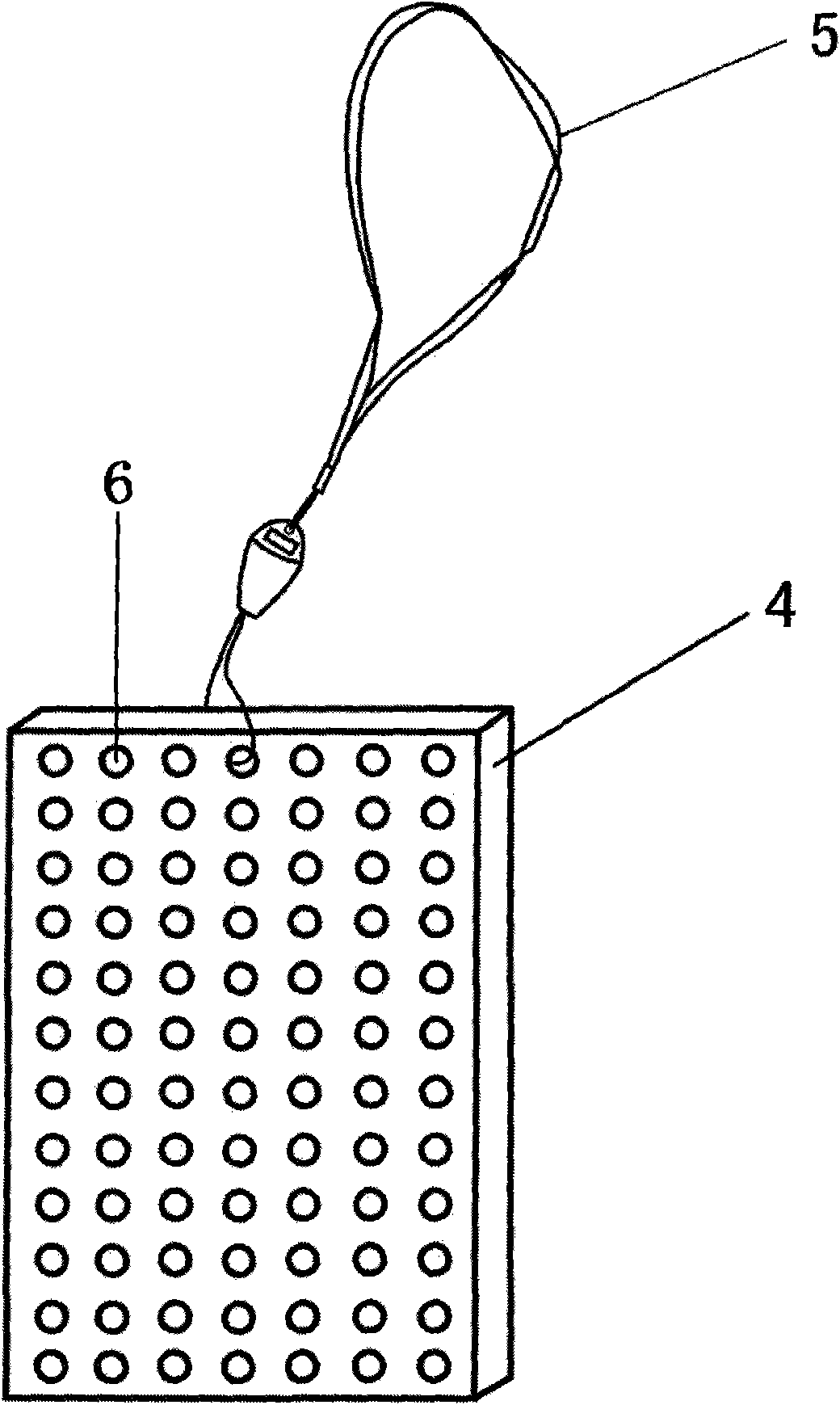
[0100]The preparation method of the present invention includes: (1) taking one or more drugs, such as two or more drugs being compatible; (2) adding 1-10% of the adjuvant by weight of the drug, and the adjuvant is ultra-fine electrical Stone powder or ultra-fine titanium dioxide powder, particle size 100nm-10um, these two accessories are available in the market. The addition of the auxiliary materials in the present invention is more ideal for the preparation effect after the auxiliary materials are added. If these auxiliary materials are not added, the effect is not as good as the addition of the auxiliary materials. ③ Put it into the mixer at room temperature and pressure to mechanically mix to make the content (2). The mixing temperature is 15°C-25°C, preferably 20°C, and the mixing time is 30-60 minutes, preferably 45 minutes. ④ Pack the content (2) into the microporous carrier material layer (3) sheet according to the weight of 1-50 grams of different specifications and ...
Embodiment 1
[0105] The raw materials, sibutramine, levothyroxine, and the auxiliary materials, tourmaline ultrafine powder (particle size: 0.2um), are prepared in a ratio of 4:5:1, put into a mixer, mixed at a temperature of 15°C-25°C, and then sent to the mixer. Put it into the powder packing machine, pack it into small bags with 0.5-1um microporous carrier non-woven material, each bag has a net weight of 5 grams, and then put it into a porous square box made of ABS material and seal it. The lanyard, that is, the obesity suspension treatment product for human trials (abbreviated as OHD).
[0106] The preparation method of OHD for animal testing is the same as that for human testing products, but the net weight of each package is 0.5 grams.
[0107] The product of this embodiment is observed on the effect of reducing weight and lipid-lowering in rats, and the observation data on the therapeutic effect of obesity in humans are as follows:
[0108] A. Observation on the effect of OHD on we...
Embodiment 2
[0147] Take the raw material omeprazole, clarithromycin, bismuth citrate, and the extract of Ligustrum lucidum, and the auxiliary material tourmaline powder (particle size: 0.2um), prepare the ingredients according to the ratio of 2:2:2:3:1, and put them into the mixer. , carry out ordinary mixing at a temperature of 15℃-25℃, and then send it to the powder packing machine, sub-package it into small bags with 0.5-1um microporous carrier non-woven material, each bag has a net weight of 5 grams, and then put it into ABS The porous square box made of the material is sealed in the inside, and the hanging belt is fastened to make the ulcer disease suspension treatment product (abbreviated as NHD).
[0148] The clinical efficacy observation data of the above products on gastric and duodenal ulcer patients are as follows:
[0149] Clinical observation of NHD in the treatment of gastric and duodenal ulcers
[0150] 1. Case selection: 80 hospitalized patients were selected, all of whom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com