Acid addition salts of synthetic intermediates for carbapenem antibiotics and processes for preparing the same
A technology for acid addition salts and compounds, which is applied in the field of preparing carbapenem antibiotics, can solve the problems of intractable treatment, unsuitable for large-scale production on an industrial scale, low product yield and the like, and achieves the effect of easy treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
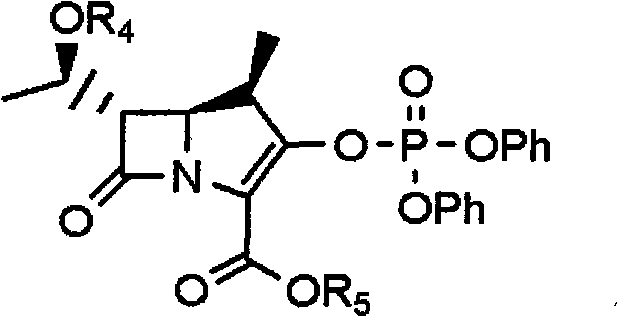
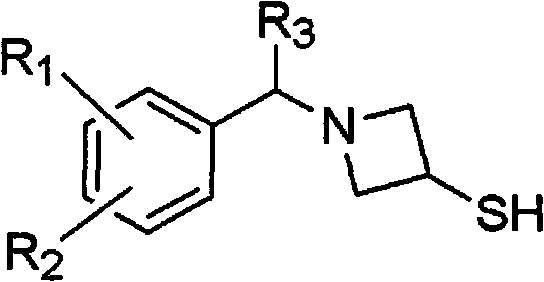
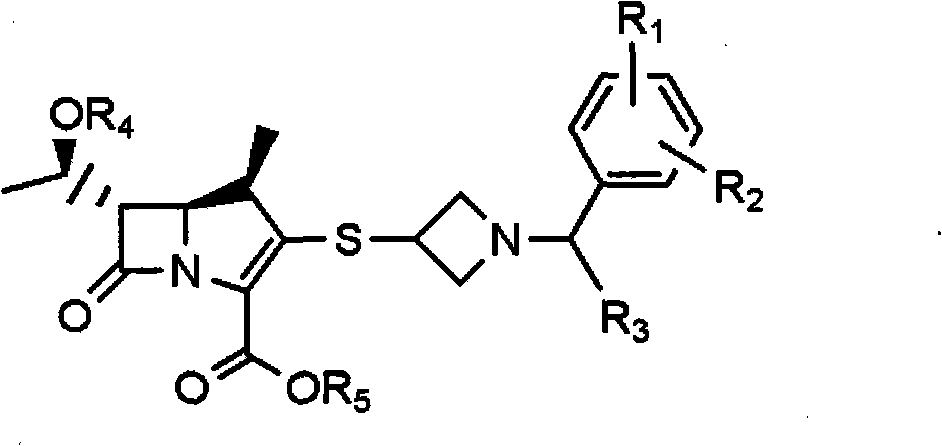
[0048] The present invention includes a method for preparing an acid addition salt of a compound of formula 3, the method comprising: (a) reacting a compound of formula 1 with a compound of formula 2; (b) adding water and an organic compound to the reaction mixture prepared in step (a) A mixed solution of solvents, acidifying the resulting mixture to a pH range between 1-5, and then separating the organic layer; (c) crystallization by adding an organic solvent to the organic layer obtained in step (b) or its concentrate:
[0049]
[0050] Among them, R 1 and R 2 are independently hydrogen, C 1 -C 3 Alkyl, C 1 -C 3 Alkoxy, halogen, hydroxyl, amino or trifluoromethyl; R 3 is hydrogen or C 1 -C 3 Alkyl; R 4 is hydrogen or a hydroxyl protecting group; R 5 is a carbonyl protecting group.
[0051] In the preparation method of the acid addition salt of the compound of formula 3 of the present invention, the hydroxyl protecting group can be a general hydroxyl protecting g...
Embodiment 1
[0094] Example 1: 1-Chloro-3-(4-fluorobenzylamine)propan-2-ol
[0095] 35Kg of 4-fluorobenzylamine was added to 500Kg of pure water, and the reaction mixture was cooled to 5°C or lower. 38.8 Kg of epichlorohydrin was slowly added to the reaction mixture while maintaining the temperature of the reaction mixture at 5°C or below. The reaction mixture was gradually heated to 20°C, stirred for 12 hours, then filtered. The obtained solid was washed with hexane and dried under reduced pressure to obtain 54.5 Kg of 1-chloro-3-(4-fluorobenzylamine)propan-2-ol (yield: 89.5%).
[0096] 1 H NMR (300MHz, CDCl 3 ) δ 2.78 (m.2H), 3.57 (d, J=5.3Hz, 2H), 3.79 (s, 2H), 3.90 (m, 1H), 7.01 (m, 2H), 7.29 (m, 2H).
Embodiment 2
[0098] Example 2: 1-Chloro-3-benzylaminopropan-2-ol
[0099] Yield: 83%: 1 H NMR (300MHz, CDCl 3 ) δ 2.72 (m, 2H), 3.56 (d, 2H), 3.80 (s, 2H), 3.86 (m, 1H), 7.32 (m, 5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com