Preparation method of Ru/C catalyst for preparing hydrogen by sodium borohydride hydrolysis
A technology for producing hydrogen by sodium borohydride and hydrolysis, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., and can solve the problem that the catalyst has short active life and cannot meet practical applications , active metal falling off and other problems, to achieve the effect of low cost, high yield and long active life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Prepare 20ml of a mixed solution with ruthenium chloride concentration of 0.06mol / L and acetic acid concentration of 0.10mol / L, add 5.0g of spherical porous carbon carrier to the above solution, mix well at room temperature, and impregnate for 24h. Discard the impregnation solution, adjust the pH value of the remaining samples to 10 with 5% NaOH solution, raise the temperature to 70°C, slowly add a 10-fold excess sodium borohydride aqueous solution with a concentration of 0.05mol / L, and react at a constant temperature of 70°C for 3h, while hot The reactant was filtered, washed with deionized water several times, and dried under vacuum at 60° C. for 3 h to obtain the desired catalyst.
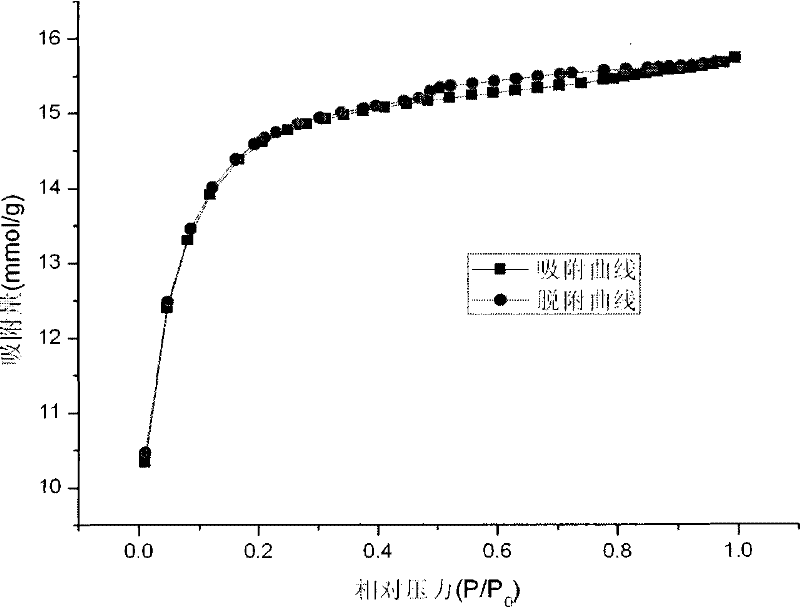
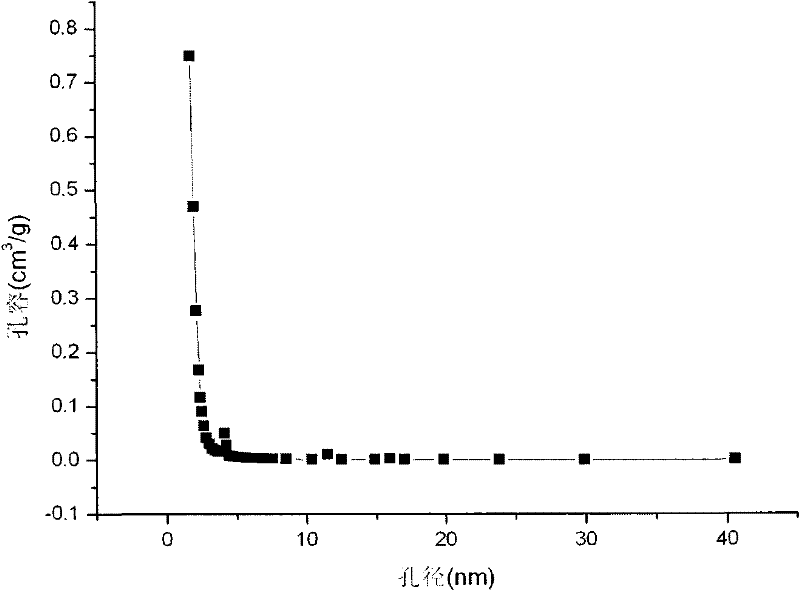
[0042] The BET curve and pore size distribution curve of the catalyst carrier used in the experiment are as follows: figure 1 and figure 2 . The product obtained in the experiment is a uniform spherical particle with a black surface, and its macroscopic photo is taken by a digital came...
Embodiment 2
[0045] Operate as in Example 1, but use 0.2 mol / L formaldehyde solution as the reducing agent, and the excess is 10 times. The deactivation curve of the catalyst prepared under this condition and the catalyst prepared in Example 1 in 10wt.% sodium borohydride solution is as follows Figure 9 . The deactivation curve of the catalyst prepared under this condition in 10wt.% and 15wt.% sodium borohydride solution is as follows Figure 10 .
Embodiment 3
[0047]Operate as in Example 1, change the reduction pH value to 11, and use 0.2 mol / L formaldehyde solution as the reducing agent, and the excess is 10 times. The deactivation curve of the catalyst prepared under this condition and the catalyst prepared in Example 2 in 10wt.% sodium borohydride solution is as follows Figure 11 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com