Method for amplifying and multiplying T cells with antigenic specificity
A specific and cell-based technology, applied in animal cells, antibacterial drugs, vertebrate cells, etc., can solve the problem that the number of effective target cells is small, the treatment cannot be satisfied, and immunogenic antigens are difficult to stimulate the specific proliferation of immune cells, etc. problem, to achieve good amplification effect, low cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Preparation of bispecific T cells (anti-tumor-specific and anti-influenza virus-specific T cells)
[0034] 1. Preparation of packaging cell line expressing recombinant virus (containing gene encoding anti-melanoma gp100TCR protein)
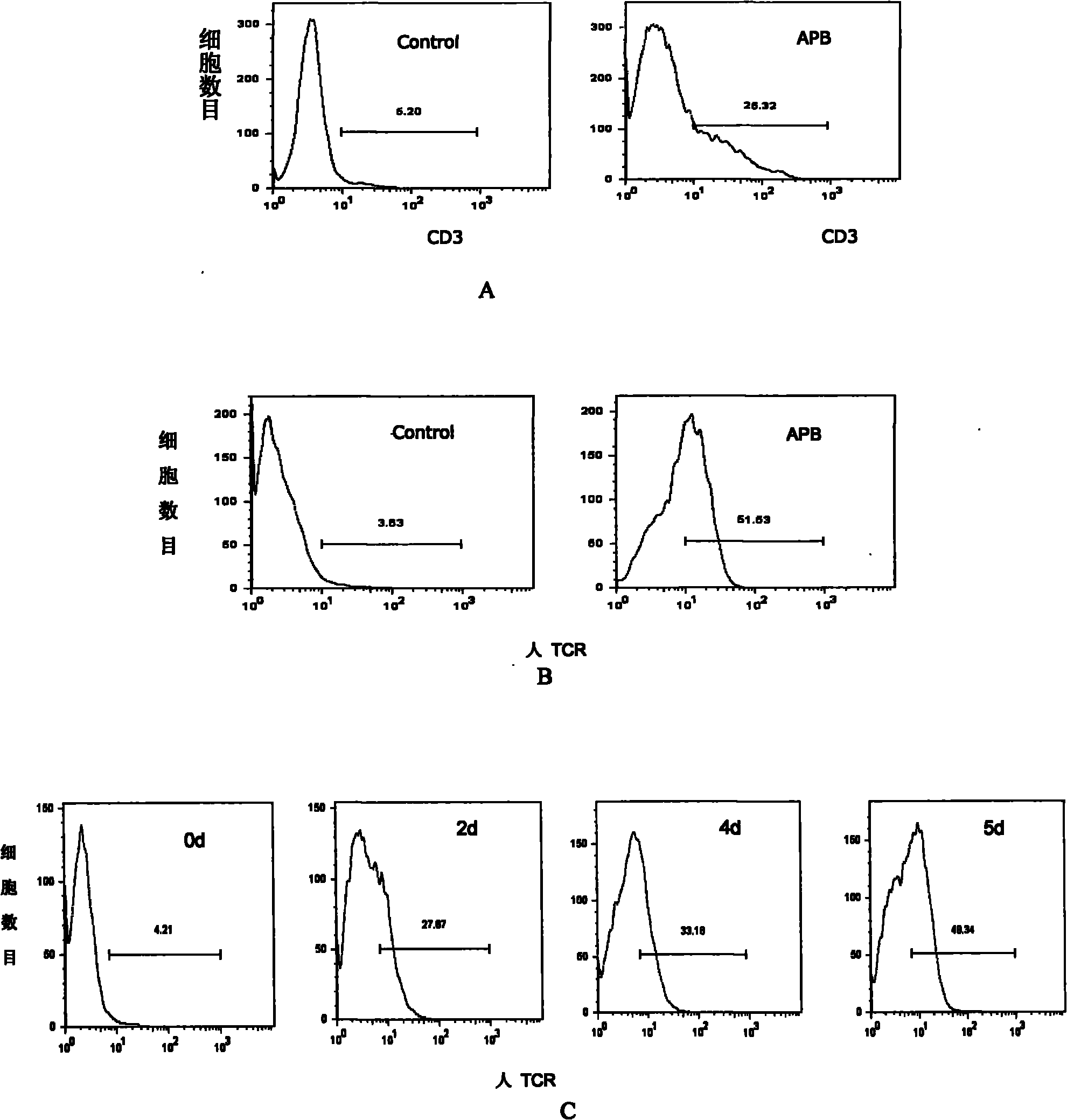
[0035] Phoenix-Eco cells were transfected with APB plasmid, and the resulting virus supernatant was used to infect PT67 packaging cells. The monoclonal PT67 virus supernatants after infection were respectively infected with SupT1 human lymphoma cell line, and the expression of human CD3 was detected. Any infected SupT1 human lymphoma cell line is a blank control. This method is used to screen for packaging cell lines that produce the highest viral titers.
[0036] The result is as figure 1 As shown in A, it shows that the screened PT67 packaging cell line produces the highest virus titer (that is, it can maximize the expression of human CD3 on the surface of SupT1 cells), and can be used as a packaging cell line for toxin produ...
Embodiment 2
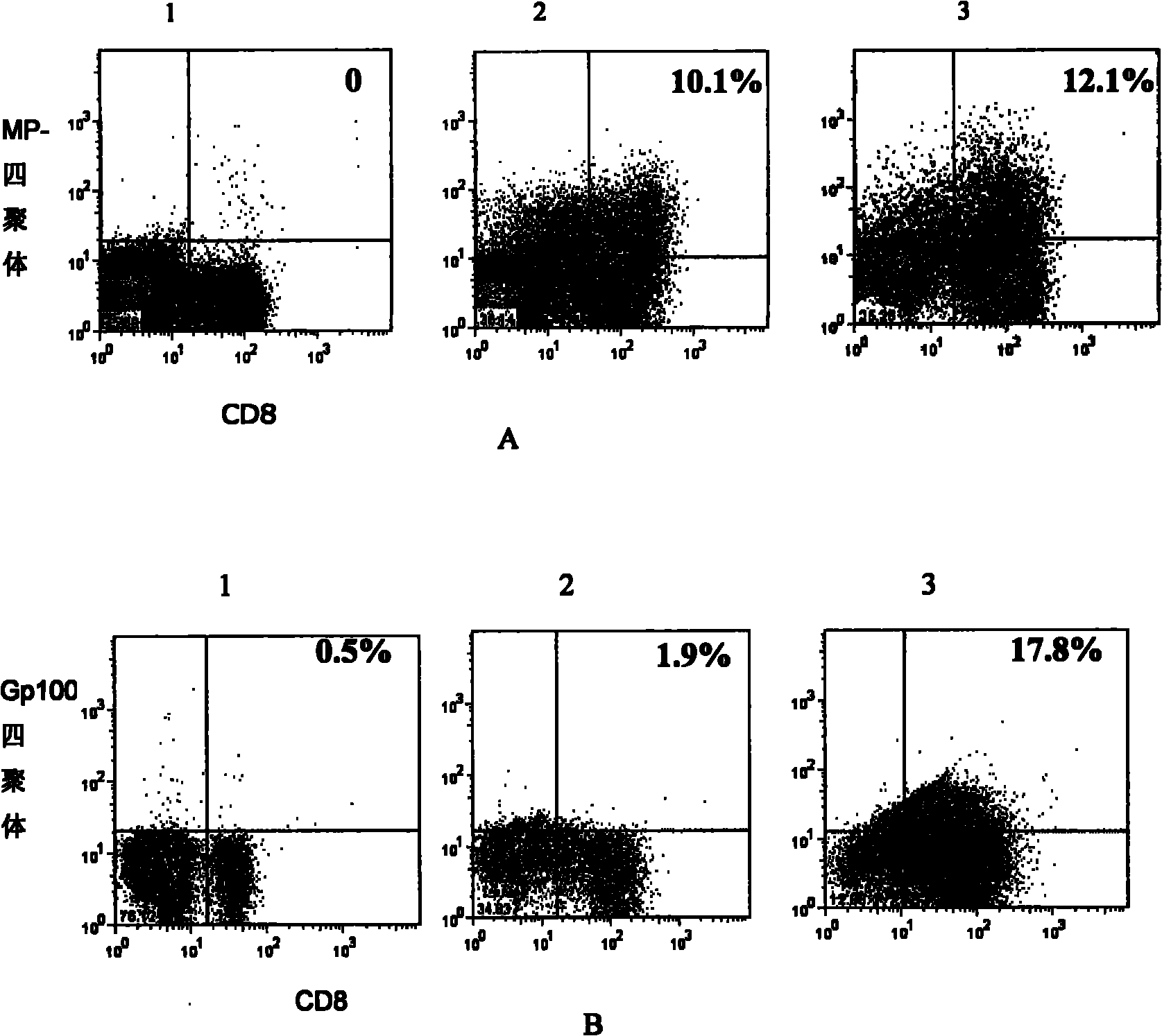
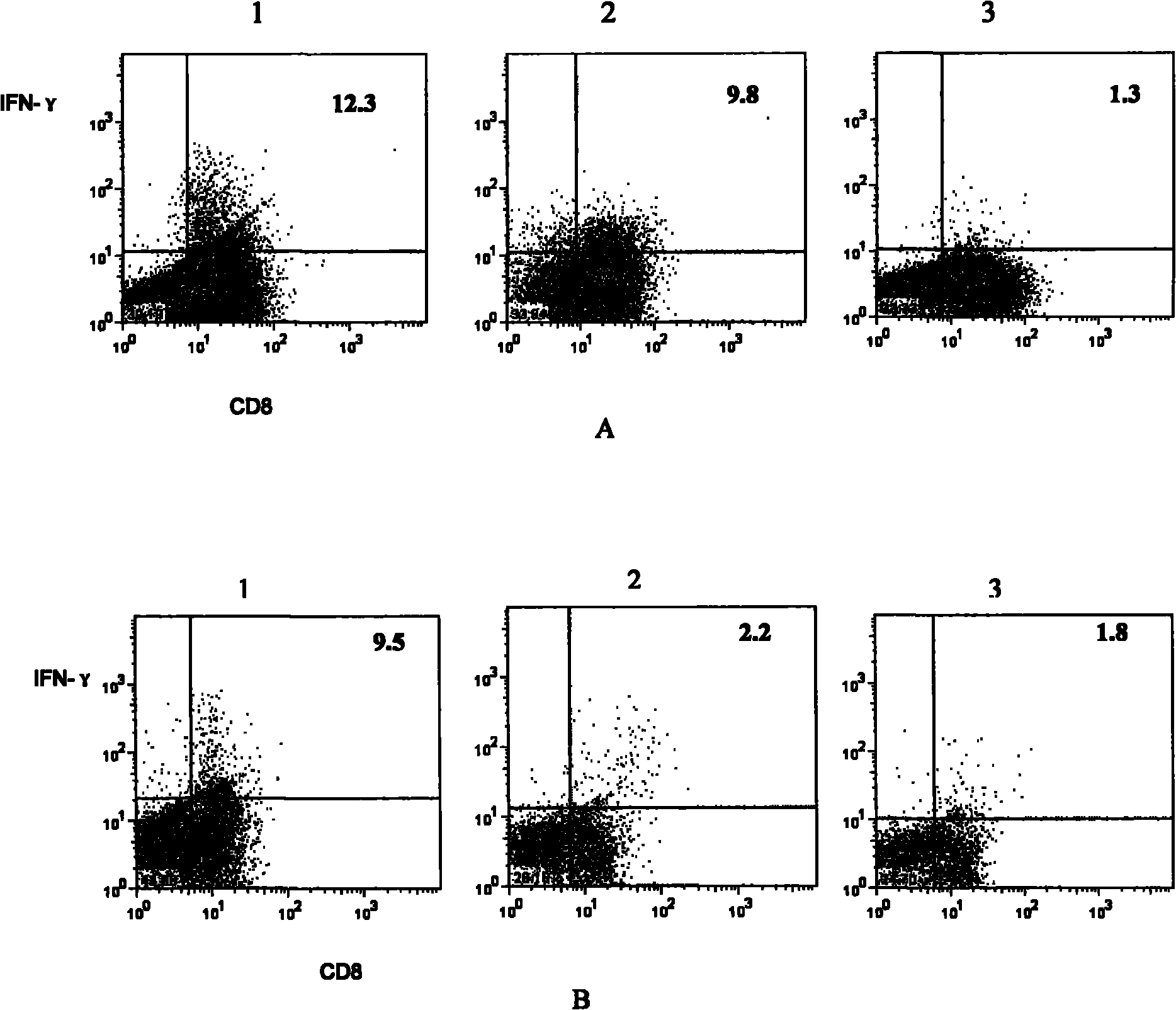
[0048] Example 2. Proliferation and function of bispecific T cells
[0049] The bispecific T cells obtained in Example 1 were subjected to the following experiments.
[0050] Tetramers of melanoma antigen gp100 peptides were as described in the literature (Estcourt, M.J., A.J. McMichael, and T. Hanke, Altered primary CD8+ T cell response to a modified virus Ankara (MVA)-vectored vaccine in the absence of CD4+ T cell help. Prepared as described in Eur J Immunol, 2005. 35(12): p.3460-7).
[0051] Tetramers of the influenza virus antigen matrix protein MP peptide were prepared according to the literature (Estcourt, M.J., A.J. McMichael, and T. Hanke, Altered primary CD8+ T cell response to a modified virus Ankara (MVA)-vectoted vaccine in the absence of CD4+ T cell Prepared as described in help. Eur J Immunol, 2005. 35(12): p. 3460-7).
[0052] FITC-labeled anti-mouse CD8 was purchased from BD, PharMingen, CA, USA;
[0053] Anti-mouse IFN-γ-PE was purchased from eBioscience, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com