Controlled-release recombinant human bone morphogenic protein-2 poly-L-lactic acid microspheres and preparation method thereof
A morphogenetic protein and lactic acid technology, which is applied to peptide/protein components, bone diseases, drug combinations, etc., can solve the problems of short release period, protein inactivation and denaturation, and difficult removal of organic solvents, and achieve the effect of mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
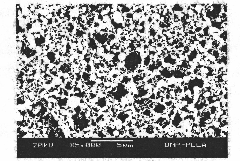
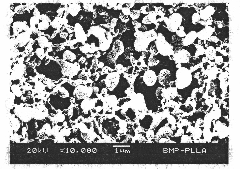
[0019] As an embodiment of the present invention, a sustained-release recombinant human bone morphogenetic protein-2 poly-L-lactic acid microsphere, the microsphere matrix component is poly-L-lactic acid, and the encapsulated drug is recombinant human bone morphogenetic protein-2, poly-L-lactic acid The mixed organic solution with recombinant human bone morphogenetic protein-2 is subjected to supercritical fluid carbon dioxide extraction solvent to separate out the microsphere crystals, the particle diameter of the microspheres ranges from 825nm to 1280nm, and the encapsulation efficiency and drug loading capacity are respectively (98.7 ±1.2)% and [(24.0±0.1)×10 -3 ]%, the average drug release concentration in vitro was (3.1±0.54) μg / mL, 4.97%±1.8% during the burst release period, and the release rate reached 92.99±7.01% after 26 days, and the released recombinant human bone morphogenetic protein-2 The active protein has a complete structure and exists in the form of homodimer...
Embodiment 1
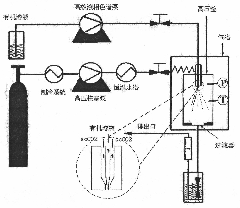
[0025] Embodiment 1 (as figure 1 shown):
[0026] 1) CO from the cylinder 2 After being liquefied by the refrigeration system, it is pressurized by a high-pressure plunger pump, heated by a constant temperature water bath in the pipeline, and pumped into a 500ml autoclave through the outer channel of the coaxial two-flow nozzle on the top of the autoclave. When the required pressure is reached in the kettle, maintain the CO 2 Pump in at a rate of 300ml / min, open the vent valve to vent at a certain rate, and keep the pressure inside the kettle constant at 12MPa. Adjust the temperature of the external drying oven and pipeline water bath of the autoclave to keep the temperature inside the autoclave constant at 33°C. When the temperature required for the experiment was 33°C, 12 mg of recombinant human BMP-2 (rhBMP-2) was dissolved in 1.2 ml of dimethyl sulfoxide, and 480 mg of poly-L-lactic acid (PLLA) was dissolved in 58.8 ml In dichloromethane (DCM), when the two solutions are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com