Method for preparing spherical super-paramagnetic ferroferric oxide nano-clusters
A ferroferric oxide and superparamagnetic technology, which is applied in nanostructure manufacturing, iron oxide/iron hydroxide, nanotechnology, etc., to achieve good reproducibility, strong magnetization, and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1. Preparation of spherical superparamagnetic ferric oxide nanoclusters
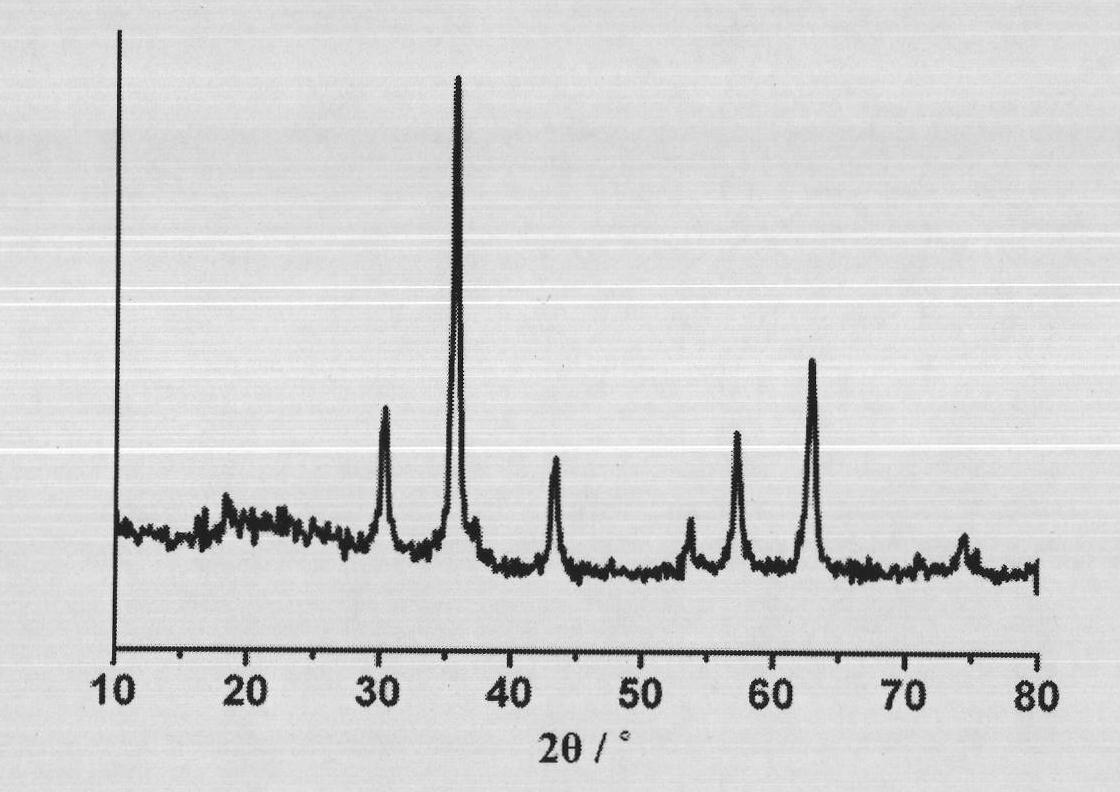
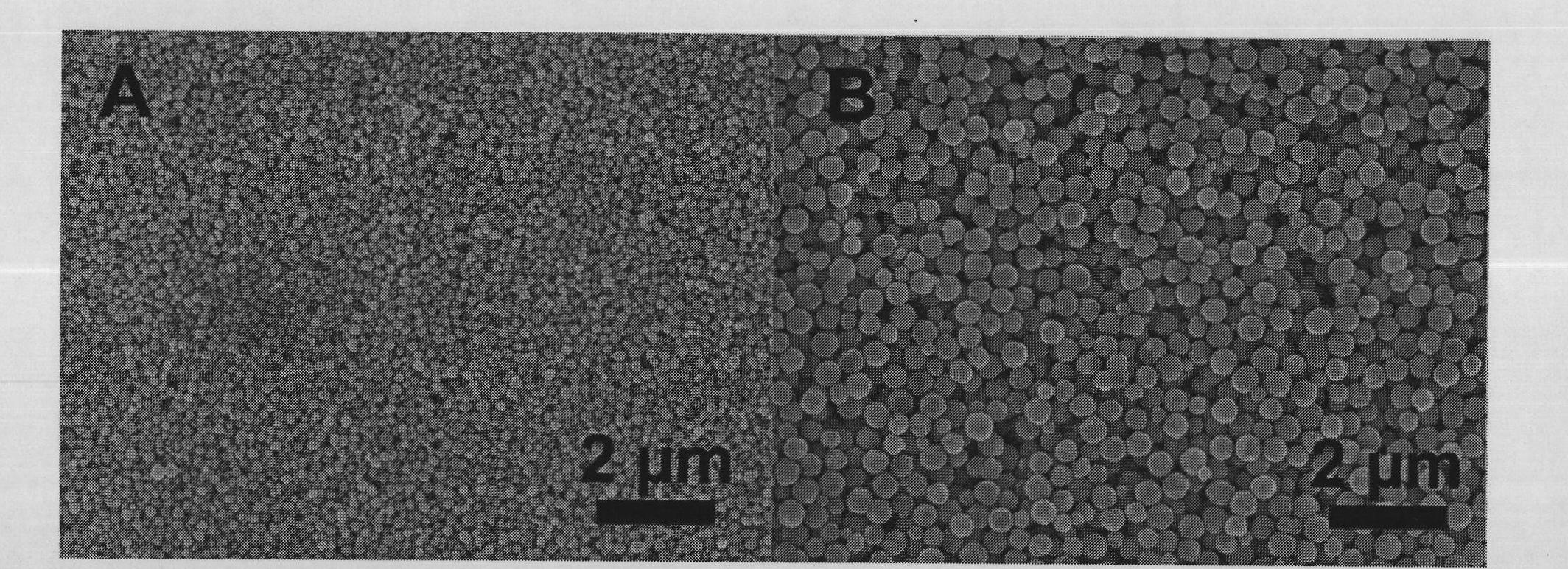
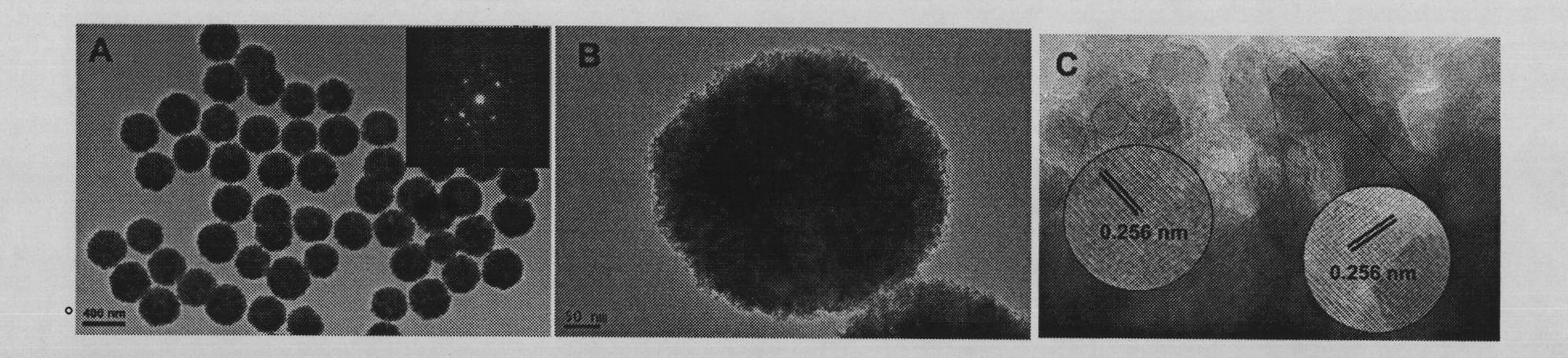
[0020] Take 5 mmol of ferric chloride hexahydrate, dissolve it in 40 ml of ethylene glycol, and stir to obtain a uniform reddish-brown solution. Take 20mmol of anhydrous sodium acetate and 1ml of PAA (number-average molecular weight: 100,000) and dissolve in the above solution in batches, sonicate and stir to obtain a uniform red viscous solution. The above solution was transferred to a hydrothermal tank with a total volume of 50ml (made of polytetrafluoroethylene), and then reacted at 220°C for 6 hours after airtight, and cooled to room temperature naturally. The sample was taken out and washed with water and alcohol for several times to obtain the product. The diameter is about 500nm, and the XRD characterization is as follows figure 1 As shown, the SEM characterization as figure 2 As shown in B, the TEM characterization is as follows image 3 As shown, the infrared characterizati...
Embodiment 2
[0021] Example 2. Preparation of Spherical Superparamagnetic Iron Tetroxide Nanoclusters
[0022] The "ethylene glycol" in Example 1 was changed to "diethylene glycol", and the other preparation conditions were the same as in Example 1 to obtain a product similar in appearance and properties to Example 1. Result is with embodiment 1.
Embodiment 3
[0023] Example 3. Preparation of Spherical Superparamagnetic Iron Tetroxide Nanoclusters
[0024] The "5mmol ferric chloride hexahydrate" in Example 1 was changed to "40mmol ferric chloride hexahydrate", and the other preparation conditions were the same as in Example 1 to obtain a product similar in appearance and properties to Example 1. Result is with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com