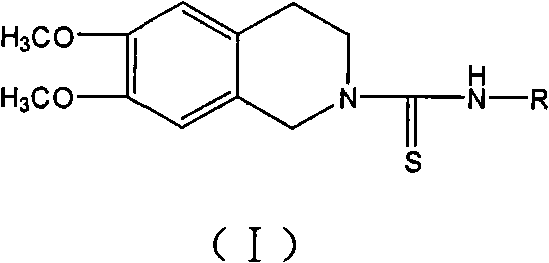
Use of tetrahydroisoquinoline derivatives
A tetrahydroisoquinoline, a technology for use, applied in the field of natural medicine and medicinal chemistry, can solve the problems of loss of response to harmful stimuli, burning sensation, loss of pain sensation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of 6,7-dimethoxytetrahydroisoquinoline (2)
[0065] Add 10 g of 3,4-dimethoxyphenethylamine and 4.6 g of formaldehyde aqueous solution into a 50 ml round bottom bottle, heat to 100° C., and keep it warm for half an hour. The reaction solution was divided into two layers, part of the upper aqueous solution was sucked off with a dropper, about 29.7 ml of 23% HCl was added, and stirred to obtain a brownish-yellow clear solution. The water was distilled off under reduced pressure to obtain a brown-yellow viscous solid, which was recrystallized with 95% ethanol to obtain 9.07 g of white crystals. Put the solid into a beaker, add ammonia water, adjust the pH to 9-10, add water, extract 3 times with chloroform, combine the chloroform layers, wash with saturated sodium chloride, anhydrous MgSO 4 dry. The liquid was filtered off and evaporated to dryness to obtain 7.62g of a light yellow solid with a yield of 71.8%, mp: 135-137°C
Embodiment 2
[0067] Preparation of isothiocyanates (4a-i)
[0068] 1.07ml (10mmol) of o-toluene was dissolved in 7ml of toluene, and 0.6ml of CS was added 2 And 1.4ml of triethylamine, heated to 35-40 ℃ for 6h, a light yellow solid appeared, filtered out the solid, washed with a small amount of toluene. The solid was dissolved in 10ml CHCl 3 , add 1.4ml triethylamine, cool in ice bath to below 0℃, add dropwise 0.78ml ClCOOCH 3 / 3mlCHCl 3 solution. React at room temperature for 5 hours, add 3ml 1N HCl, stir for 10 minutes, pour the reaction solution into a separatory funnel, and separate the CHCl 3 layer, washed with water to neutral, anhydrous Na 2 SO 4 dry. The solid was filtered out, and the filtrate was subjected to column chromatography (PE was the eluent) to obtain a colorless liquid, which was o-methylphenyl isothiocyanate 4a.
[0069] The same method makes p-methylphenyl isothiocyanate (4b), p-methoxyphenyl isothiocyanate (4c), m-methoxyphenyl isothiocyanate (4d), o- Methox...
Embodiment 3
[0071] 6,7-dimethoxy-2-[N-(2-methylphenyl)]thioureido-1,2,3,4-tetrahydroisoquinoline (I 1 ) preparation
[0072] Add 20ml of absolute ethanol and 0.27ml (2mmol) o-methylphenyl isothiocyanate (4a) to 0.39g (2mmol) of 2, and reflux until the reaction is complete. The reaction solution was left to cool down, and solids precipitated out. The solids were filtered out, washed with absolute ethanol, and dried to obtain 0.42 g of white solids, yield 61.4%, mp: 143-145°C
[0073] IR (KBr, cm -1 ): 3241 (v NH ), 3010(v φH ), 1611 (v C=C ), 1517 (v C=S ), 1493, 1474 (aromatic), 1322, 1180, 731
[0074] 1 HNMR (CDCl 3 , 300Hz): δ7.13-7.27 (m, 4H, Ar-H), δ6.96 (bs, 1H, NH), δ6.61-6.68 (d, 2H, Ar-H), δ4.85 (s , 2H, ArCH 2 N), δ3.99(t, 2H, ArCH 2 C H 2 N), δ3.86(d, 6H, OCH 3 , OCH 3 ), δ2.88(t, 2H, ArC H 2 CH 2 N), δ2.29(s, 3H, CH 3 )
[0075] MS (ESI, m / z): 343.2 (M+H + , base peak)
[0076] Anal.Calcd.for C 19 h 22 N 2 SO 2 : C 66.67, H 6.43, N 8.19; Found C 66.48...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com