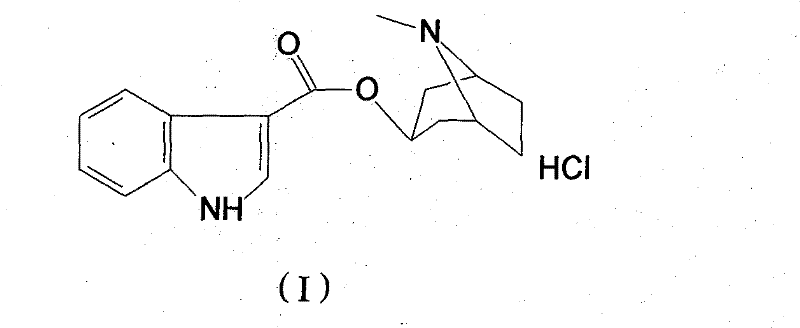
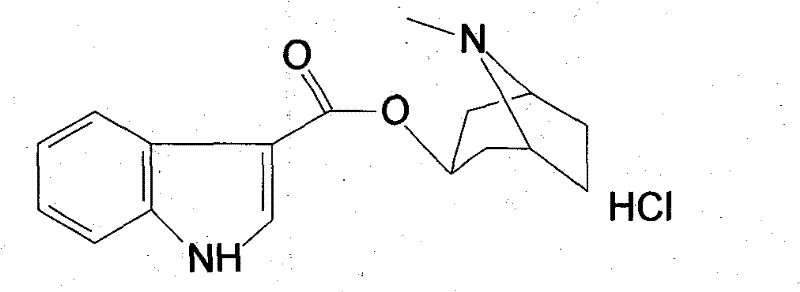
High-purified tropisetron hydrochloride compound
A technology for tropisetron hydrochloride and tropisetron, which is applied in the field of medicine, can solve the problems of low purity of finished products and affect the safety of pharmaceutical preparations, and achieves the effects of high yield, low cost and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The refining of embodiment 1 tropisetron hydrochloride
[0019] (1) 50g tropisetron hydrochloride crude product is dissolved in 500ml water, then slowly add 10% (g / ml) sodium carbonate solution, stir and react until the solution pH is 8, produce tropisetron precipitation, filter, 50 ℃ Drying under reduced pressure gave 41.7 g of tropisetron.
[0020] (2) Dissolve 41.7 g of Tropisetron obtained in the previous step in 300 ml of n-butanol, add 3.5 g of activated carbon, stir at room temperature for 35 minutes, filter for decarburization, and collect the filtrate.
[0021] (3) the filtrate obtained in the previous step is utilized for separation and purification by a preparative chromatographic column to obtain tropisetron hydrochloride refined product, wherein the mobile phase used in the chromatographic column is that the volume accounts for mobile phase 35% dichloromethane and the volume accounts for mobile phase 65% Hydrochloric acid aqueous solution with a pH of 1; t...
Embodiment 2
[0029] The refining of embodiment 2 tropisetron hydrochloride
[0030] (1) 50g tropisetron hydrochloride crude product is dissolved in 500ml water, then slowly add the sodium hydroxide solution of 8% (g / ml), stirring reaction is 9 to solution pH, produces tropisetron precipitation, filters, 40 °C and dried under reduced pressure to obtain 41.3 g of tropisetron.
[0031] (2) Dissolve 41.3 g of Tropisetron obtained in the previous step in 300 ml of n-hexane, add 3.3 g of activated carbon, stir at room temperature for 45 minutes, filter for decarburization, and collect the filtrate.
[0032] (3) the filtrate that last step is obtained utilizes preparative chromatographic column to carry out separation and purification to obtain tropisetron hydrochloride refined product, wherein the mobile phase that chromatographic column uses is the dichloromethane that volume accounts for mobile phase 45% and volume accounts for mobile phase 55% Hydrochloric acid aqueous solution with a pH of ...
Embodiment 3
[0040] The refining of embodiment 3 tropisetron hydrochloride
[0041] (1) 50g tropisetron hydrochloride crude product is dissolved in 500ml water, then slowly add the sodium bicarbonate solution of 12% (g / ml), stirring reaction is 7 to solution pH, produces tropisetron precipitation, filters, 40 °C and dried under reduced pressure to obtain 42.0 g of tropisetron.
[0042] (2) Dissolve 42.0 g of Tropisetron obtained in the previous step in 300 ml of n-hexane, add 3.2 g of activated carbon, stir at room temperature for 40 minutes, filter for decarburization, and collect the filtrate.
[0043](3) the filtrate that last step is obtained utilizes preparative chromatographic column to carry out separation and purification to obtain tropisetron hydrochloride refined product, wherein the mobile phase that chromatographic column uses is the dichloromethane that volume accounts for mobile phase 40% and volume accounts for mobile phase 60% Hydrochloric acid aqueous solution with a pH o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com