Synthetic method of maleimide phosphatidyl ethanolamine
A technology of hydroxysuccinimide and compound is applied in the field of preparation of maleimidized phosphatidylethanolamine, and can solve the problems of difficulty in separation and purification of by-products, time-consuming and laborious problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
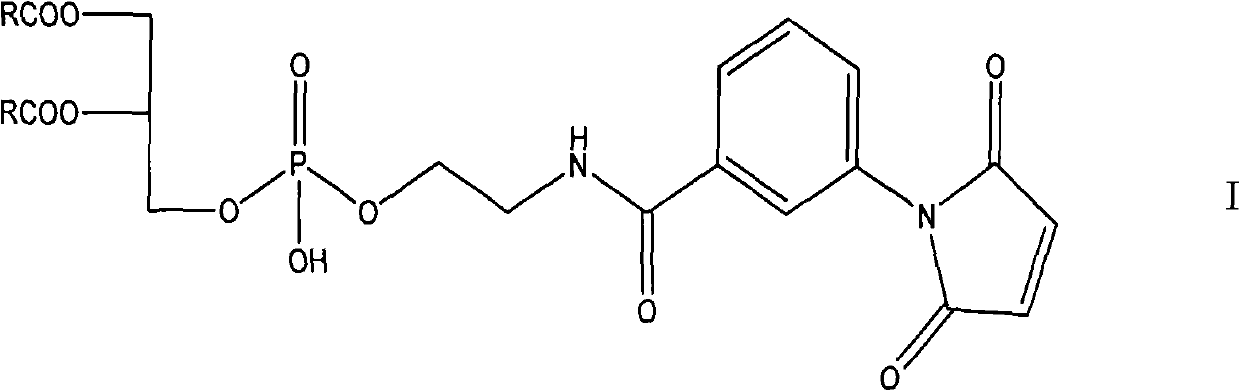
[0054] 1. The preparation method provided by the invention has a high yield of the compound of formula I;
[0055] 2. The preparation method provided by the invention saves time;
[0056] 3. The preparation method provided by the invention reduces the cost.
Embodiment 1
[0061] Preparation of Formula I Compound A
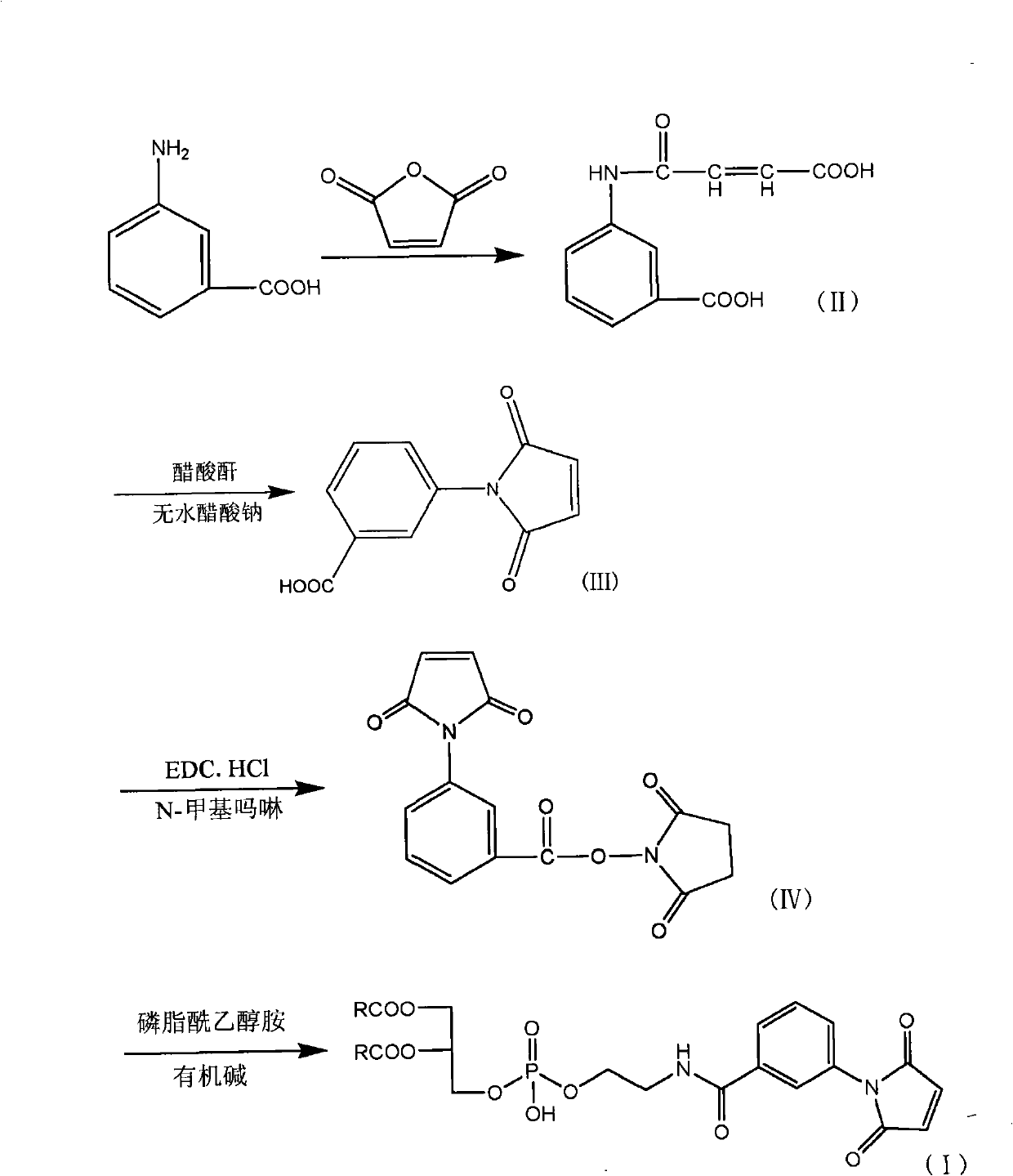
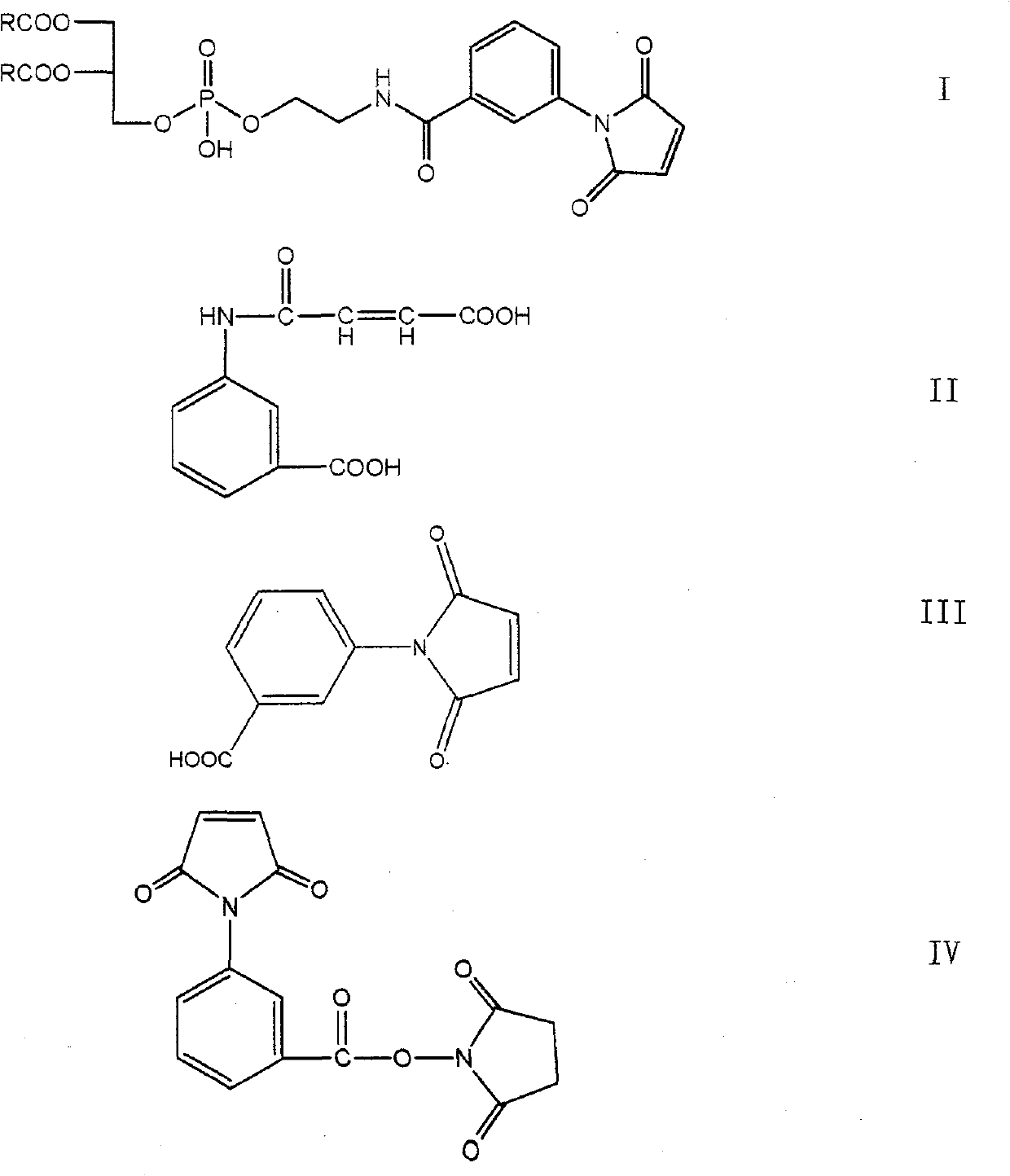
[0062] The first step: Add 300ml tetrahydrofuran, m-aminobenzoic acid (20g, 0.146mol) and maleic anhydride (15.7g, 0.166mol) into a 500ml three-necked flask, react at room temperature for 1 hour, and filter with a Buchner funnel to obtain a light yellow solid , and dried to obtain 28.76g of the compound of formula II, with a yield of 87.40%, m.p.: 206-208°C.
[0063] The second step: add 150ml of acetic anhydride, the formula II compound (15g, 0.0638mol) and anhydrous sodium acetate (7.5g, 0.091mol) obtained in the first step to a 250ml three-necked bottle, and stir at a high speed in an oil bath at 90°C for 3h , the solution turned dark yellow, the reaction solution was poured into ice water containing 300ml of water, stirred for 3h, a yellow precipitate was produced, the crude product of the compound of formula III was obtained by filtration, and 11.35g of the compound of formula III was obtained by recrystallization with absolute...
Embodiment 2
[0071] Preparation of Formula I Compound B
[0072] The first step: add 300ml tetrahydrofuran, m-aminobenzoic acid (20g, 0.146mol) and maleic anhydride (21.4g, 0.218mol) into a 500ml three-necked flask, react at room temperature for 1h, and filter with a Buchner funnel to obtain a light yellow solid , and dried to obtain 31.22g of the compound of formula II, with a yield of 91.40%, m.p.: 206-208°C.
[0073] The second step: add 100ml of acetic anhydride, the compound of formula II obtained in the first step (15g, 0.0638mol) and anhydrous sodium acetate (5.21g, 0.064mol) in a 250ml three-necked bottle, and stir at a high speed in an oil bath at 90°C for 3h. The solution turned dark yellow, and the reaction solution was poured into ice water containing 300ml of water, stirred for 3 hours, a yellow precipitate was produced, filtered to obtain the crude compound of formula III, and recrystallized with absolute ethanol to obtain 10.38 g of compound of formula III, with a yield of 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com