Perovskite type composite oxide catalyst for purifying tail gas of internal combustion engine
A technology of composite oxides and catalysts, applied in the direction of metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problem of low NO conversion rate and unwillingness to mention the conversion rate and other issues to achieve the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, La 0.8 Cu 0.2 Ni 0.8 mn 0.2 o 3 Catalyst activity
[0025] La(NO 3 ) 3 ·6H 2 O, Cu(NO 3 ) 2 ·3H 2 O, Ni(NO 3 ) 2 ·6H 2 O and Mn(NO 3 ) 2 4H 2 Dissolve O in water to prepare a mixed solution with a total concentration of metal ions of 0.2mol / L. Take citric acid according to 0.6 times the total number of moles of metal ions and add it to the mixed solution. After stirring and dissolving, add ammonia water dropwise to adjust the pH of the mixed solution. The value is about 8.5, constant temperature is 70°C, electric stirring until it becomes a wet gel, dried at 110°C for 12 hours, cooled, and the formed xerogel is heated to about 350°C on an electric furnace, and the xerogel spontaneously ignites to obtain gray-black ash , grind the ashes into powder, and bake them at 750°C for 2 hours to become La 0.8 Cu 0.2 Ni 0.8 mn 0.2 o 3 composite oxides.
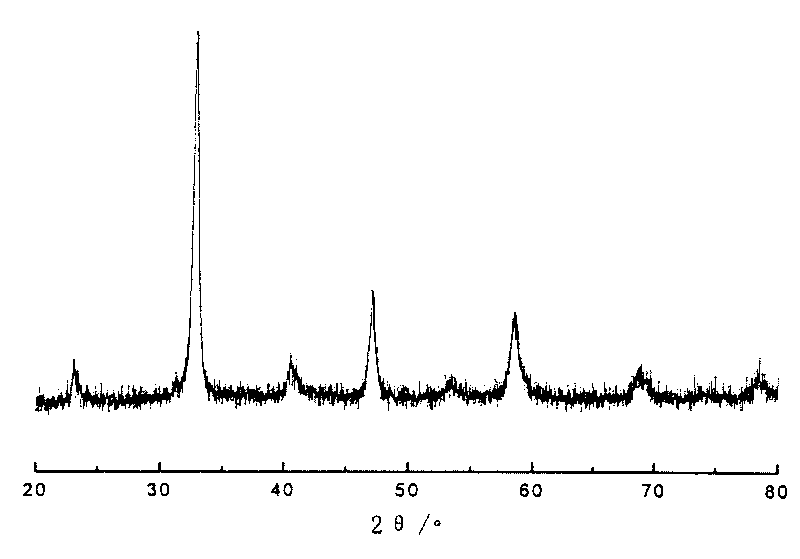
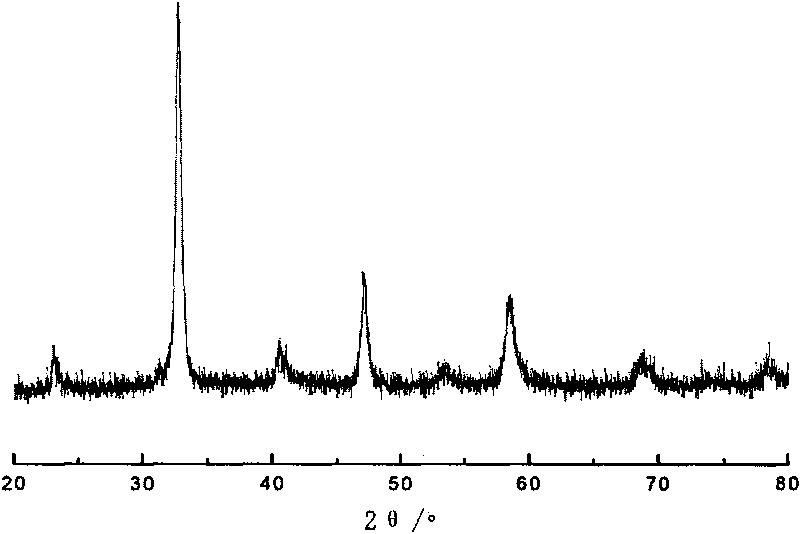
[0026]Catalyst structure confirmation: the composite oxide was subjected to X-ray diffractio...
Embodiment 2
[0030] Example 2, La 0.8 Cu 0.2 NiO 3 Catalyst activity
[0031] La(NO 3 ) 3 ·6H 2 O, Cu(NO 3 ) 2 ·3H 2 O and Ni(NO 3 ) 2 ·6H 2 Dissolve O in water to prepare a mixed solution with a total concentration of metal ions of 0.2mol / L. Take citric acid according to 0.6 times the total number of moles of metal ions and add it to the mixed solution. After stirring and dissolving, add ammonia water dropwise to adjust the pH of the mixed solution. When the value is about 8.5, keep the temperature at 70°C, stir with electric motor until it becomes a wet gel, dry it at 110°C for 12 hours, cool it, and heat the formed xerogel to about 350°C on an electric furnace, and the xerogel spontaneously ignites to get gray-black ash , grind the ashes into powder, and bake them at 750℃2 for 2 hours to become La 0.8 Cu 0.2 NiO 3 composite oxides.
[0032] The composite oxide also forms a perovskite cubic crystal structure, the La element and the Cu element are located at the A site of t...
Embodiment 3
[0036] Example 3, La 0.8 Cu 0.2 Ni 0.8 Fe 0.2 o 3 Catalyst activity
[0037] La(NO 3 ) 3 ·6H 2 O, Cu(NO 3 ) 2 ·3H 2 O, Ni(NO 3 ) 2 ·6H 2 O and Fe(NO 3 ) 3 9H 2 Dissolve O in water to prepare a mixed solution with a total concentration of metal ions of 0.2mol / L. Take citric acid according to 0.6 times the total number of moles of metal ions and add it to the mixed solution. After stirring and dissolving, add ammonia water dropwise to adjust the pH of the mixed solution. The value is about 8.5, electric stirring at a constant temperature of 70°C until it becomes a wet gel, dried at 110°C for 12 hours, cooled, and the formed xerogel is heated to about 350°C on an electric furnace, and the xerogel spontaneously ignites to obtain gray-black ash. Grind the ashes into powder and bake them at 750°C for 2 hours to become La 0.8 Cu 0.2 Ni 0.8 Fe 0.2 o 3 composite oxides.
[0038] The composite oxide also forms a perovskite cubic crystal structure, La and Cu element...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com