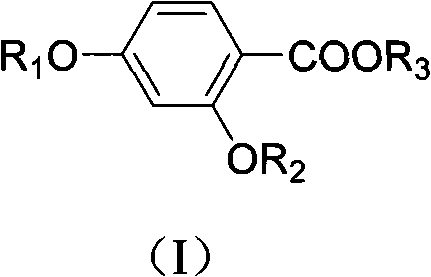
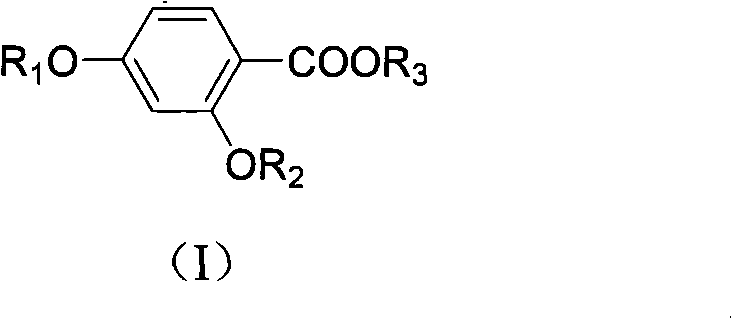
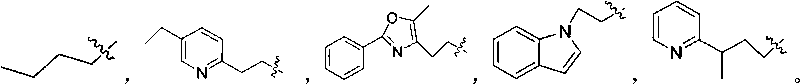
Salicylic acid compounds with insulin-sensitizing activity and preparation method thereof
A kind of compound and acid technology, applied in the field of salicylic acid compound and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Synthesis of 2-acetoxy-4-n-butoxybenzoic acid (compound 1)
[0083] Step 1: Suspend 5 g of 2,4-dihydroxybenzoic acid in a mixture of acetic anhydride and acetic acid, add mixed acid [98% sulfuric acid: 37% hydrochloric acid=1:1] (V:V), seal and heat up Reaction at 85°C for 0.5h, hot suction filtration, the filtrate was cooled in an ice bath to precipitate solids, filtered, and the filter residue was dried under reduced pressure at 55°C;
[0084] Add anhydrous potassium carbonate and dry N,N-dimethylformamide to the above filter residue, add benzyl bromide dropwise, react at room temperature for 2 hours, filter, add ethyl acetate to the filtrate, wash with water and saturated brine in sequence, and wash the organic layer with Dry over sodium sulfate, use petroleum ether: ethyl acetate = 3:1 (V:V) as eluent, separate by silica gel column chromatography and evaporate to dryness to obtain intermediate (II);
[0085] Wherein, the mass ratio of 2,4-dihydroxybenzoi...
Embodiment 2
[0091] Embodiment 2: the synthesis of methyl 2-acetoxy-4-n-butoxybenzoate (compound 2)
[0092] Under ice-bath conditions, add thionyl chloride dropwise to anhydrous methanol, then add 5 g of compound 1, stir at room temperature for 5 h, thin-layer chromatography (TLG) monitors the completion of the reaction, evaporate the solvent to dryness, add ethyl acetate, and successively add water, Wash with saturated brine, dry over anhydrous sodium sulfate, and evaporate the solvent to dryness;
[0093] Wherein, the mass ratio of the product obtained in the third step to anhydrous methanol, thionyl chloride and ethyl acetate is 1:39.5:2:180, respectively.
[0094] The resulting product yield is 90.2%, 1 H NMR (CDCl 3 ): δ=0.86(t, 3H), 1.29(m, 2H), 1.72(m, 2H), 2.12(s, 3H), 3.68(s, 3H), 3.90(t, 2H), 6.78(s, 1H), 6.93 (d, J=8.8Hz, 1H), 7.82 (d, J=8.8Hz, 1H).
Embodiment 3
[0095] Example 3: Synthesis of 2-acetoxy-4-[2-(5-ethyl-2-pyridine)ethoxy]benzoic acid (compound 3)
[0096] Step 1: Suspend 5 g of 2,4-dihydroxybenzoic acid in a mixture of acetic anhydride and acetic acid, add mixed acid [98% sulfuric acid: 37% hydrochloric acid=1:1] (V:V), seal and heat up Reaction at 95°C for 1.5h, hot suction filtration, the filtrate was cooled in an ice bath to precipitate solids, filtered, and the filter residue was dried under reduced pressure at 65°C;
[0097] Add anhydrous potassium carbonate and dry N,N-dimethylformamide to the above filter residue, add benzyl bromide dropwise, react at room temperature for 3 hours, filter, add ethyl acetate to the filtrate, wash with water and saturated saline in sequence, and wash the organic layer with Dry over sodium sulfate, use petroleum ether: ethyl acetate = 3:1 (V:V) as eluent, separate by silica gel column chromatography and evaporate to dryness to obtain intermediate (II);
[0098] Wherein, the mass ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com