Pravastatin sodium tablet as well as application and preparation method thereof
A technology of pravastatin sodium and tablets, which is applied in the field of medicine, can solve problems such as difficulties in tablet production and affect product quality, and achieve the effects of facilitating storage and transportation, high stability, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
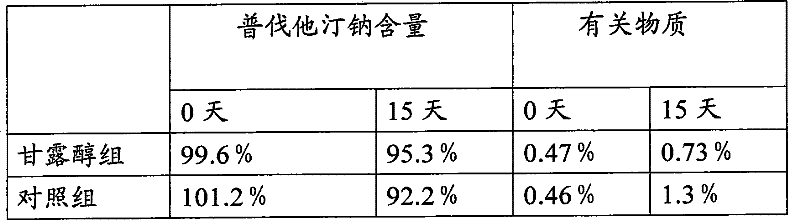
[0032] The present embodiment provides a preferred prescription of pravastatin sodium tablet of the present invention:
[0033] Pravastatin Sodium 100g
[0034] Mannitol 724g
[0035] 750g microcrystalline cellulose
[0036] PVP K30 84g
[0037] Sodium Carboxymethyl Cellulose 84g
[0038] Titanium dioxide 100g
[0041] Made into 10000 pieces
[0042] The prepared pravastatin sodium tablet has a specification of 10 mg and a clinical dosage of 10-40 mg.
Embodiment 2
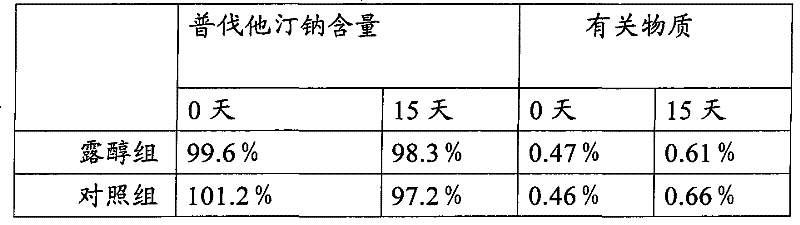
[0044] The present embodiment provides a preferred prescription of pravastatin sodium tablet of the present invention:
[0045] Pravastatin Sodium 300g
[0046] Mannitol 2172g
[0047] Microcrystalline Cellulose 2250g
[0048] PVP K30 252g
[0049] Sodium Carboxymethyl Cellulose 252g
[0050] Titanium dioxide 300g
[0052] Magnesium Stearate 51g
[0053] Made 30000 pieces
[0054] The prepared pravastatin sodium tablet has a specification of 10 mg and a clinical dosage of 10-40 mg.
Embodiment 3
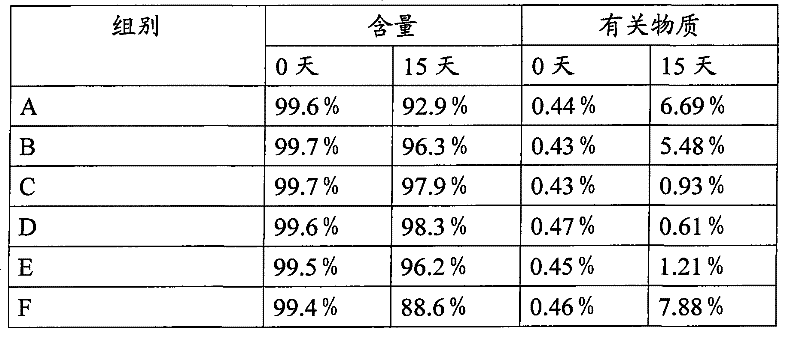
[0056] Pravastatin sodium tablet of the present invention adopts the method preparation of direct compression tablet, and its specific operation steps are as follows:
[0057] According to the formula of embodiment 1 or 2, the pravastatin sodium of the stated amount of the formula is first mixed with the magnesium oxide of the stated amount of the formula, after dropping into the HLSG20A wet mixing granulator and mixing uniformly, then adding 600 grams of mannitol and mixing, Add the remaining mannitol and mix evenly, then add microcrystalline cellulose, PVP K30, sodium carboxymethyl cellulose, and magnesium stearate in the amount specified in the formula and mix for 5 minutes, then discharge. The mixed material is directly compressed using a ZP-12 rotary tablet press. Plain tablets can be sold directly on the market, or choose Opadry 85G68918 as the coating material, and use BG-5 high-efficiency coating machine to coat the film (inlet air temperature 50°C, tablet bed temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com