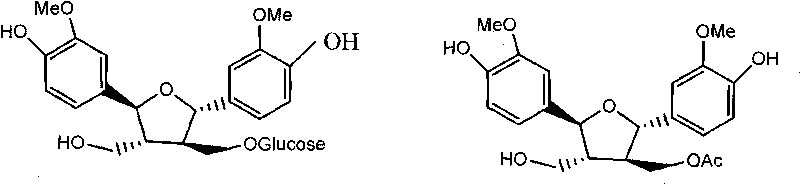
5alpha-reductase urtica open-loop lignan glycoside D inhibitor, preparation method thereof and use thereof
The technology of a reductase inhibitor and a split lignan is applied in the field of extraction of biologically active components of traditional Chinese medicine, can solve problems such as weak action, and achieve the effects of low cost and simple and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The medicinal material of split-leaf nettle is collected from Aba Prefecture, Sichuan Province. The method steps of preparing nettle split cyclolignan C from Urtica split leaf are as follows:
[0041] (1) After crushing the dried underground part of Urtica schizophyllum, it is reflux extracted with industrial ethanol with a mass concentration of 95%, the ratio of solid to liquid (kg / l)=1:8, the extraction temperature is 90°C, the extraction time is 3h, and the extraction The number of times is 3 times, the extracts are combined, filtered and concentrated under reduced pressure until there is no alcohol smell;
[0042] (2) Suspend 200 g of the ethanol extract with 2 L of hot water, and extract with 2 L of petroleum ether, ethyl acetate, and water-saturated n-butanol successively, each extracting 3 times;
[0043] (3) 30 g of the raffinate aqueous layer extract was separated by D101 macroporous resin (1 kg), eluted with an ethanol-water gradient (water → 30% ethanol → 50...
Embodiment 2
[0047] The 5-alpha reductase inhibitory effect of Urtica schizolignanside D prepared in Example 1 was tested.
[0048] 5-α reductase inhibition experimental steps are as follows:
[0049] (1) Preparation of 5-alpha reductase:
[0050]Three male SD rats, weighing 200 ± 10 g, were provided by the Experimental Animal Center of Dalian Medical University. They were fasted and killed overnight, and the ventral prostate tissue was quickly taken out and cut into pieces on an ice table. mol L -1 , calcium chloride 1.91mmol·L -1 ) was homogenized in a glass homogenizer, centrifuged at 10,000 rpm at low temperature and high speed for 20 min at 4° C., and the supernatant was centrifuged at low temperature and high speed at 16,000 rpm for 30 min to obtain the 5α-reductase extract. The protein content was determined by the lowry method, and the total protein content in the enzyme extract was used to represent the content of the 5α-reductase extract. The extract was stored in a small cent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com