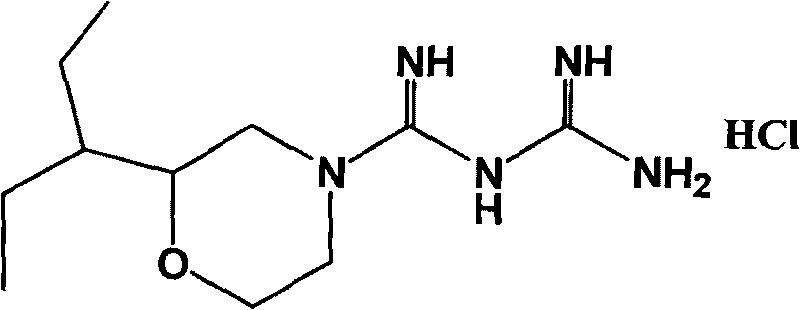
Hydrochloric acid 2-(1-ethyl propyl) moroxydine, preparation method and application thereof
A technology of ethylpropyl and morpholine guanidine, which is applied in the field of medicine, can solve the problems of unfavorable clinical application and high toxicity of morpholine guanidine hydrochloride, and achieve the effects of simple preparation method, low price and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Preparation of 2-(1-ethylpropyl) morpholine guanidine hydrochloride
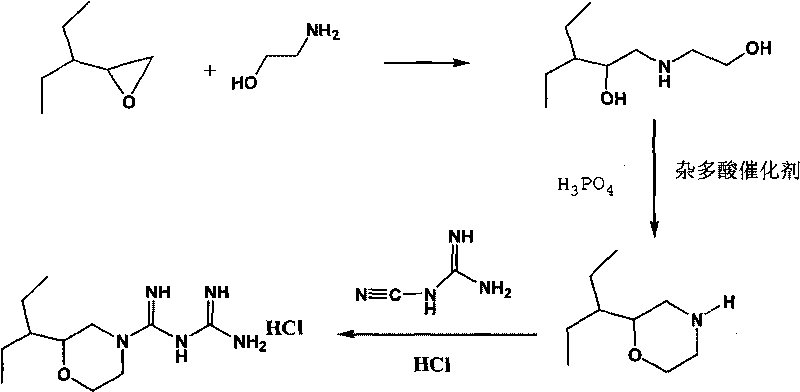
[0042] 1. Preparation of 2-(1-ethylpropyl)morpholine
[0043] Add 1mol of 2-(1-ethylpropyl)oxirane and 1mol of 2-aminoethanol into the reaction kettle, the reaction temperature is 40°C, stir, and react for 1.5 hours. After the reaction, obtain 1-ethanolamino by vacuum distillation -2-Ethyl-2-butanol.
[0044] Then add 1 mol of concentrated phosphoric acid and 1 mol of 1-ethanolamino-2-ethyl-2-butanol to the reaction kettle in sequence, stir at 50°C until the sample is completely melted, then heat to 65°C, add 3g of solid heteropoly Acid catalyst, stirred and reacted for 2 hours, opened the dehydration device to fractionate the water generated by the reaction; after the reaction was completed, cooled to room temperature, filtered; the solid heteropolyacid catalyst was recycled, and the filtrate was added to the reactor, and then 1mol 1-ethanolamino- 2-Ethyl-2-butanol neutralizes phosphor...
Embodiment 2
[0053] Embodiment 2 Preparation of 2-(1-ethylpropyl) morpholine guanidine hydrochloride
[0054] 1. Preparation of 2-(1-ethylpropyl)morpholine
[0055] Add 1mol of 2-(1-ethylpropyl)oxirane and 3mol of 2-aminoethanol into the reaction kettle, the reaction temperature is 50°C, stir, and react for 2.5 hours. After the reaction, obtain 1-ethanolamino by vacuum distillation -2-Ethyl-2-butanol.
[0056] Add 0.5 mol of concentrated phosphoric acid and 1 mol of 1-ethanolamino-2-ethyl-2-butanol to the reaction kettle in sequence, stir at 50°C until the sample is completely melted, then heat to 75°C, add 5g of solid heteropoly Acid catalyst, stirred and reacted for 3 hours, opened the dehydration device to fractionate the water generated by the reaction; after the reaction was completed, cooled to room temperature, filtered; the solid heteropolyacid catalyst was recycled, and the filtrate was added to the reactor, and then 0.5mol 1-ethanolamino- 2-Ethyl-2-butanol neutralizes phosphori...
Embodiment 3
[0059] Embodiment 3 Preparation of 2-(1-ethylpropyl) morpholine guanidine hydrochloride
[0060] 1. Preparation of 2-(1-ethylpropyl)morpholine
[0061] 2mol 2-(1-ethylpropyl)oxirane and 10mol 2-aminoethanol were added to the reaction kettle, the reaction temperature was 70°C, stirred, and reacted for 3.5 hours. After the reaction, 1-ethanolamino was obtained by vacuum distillation -2-Ethyl-2-butanol.
[0062] Add 0.5 mol of concentrated phosphoric acid and 1.5 mol of 1-ethanolamino-2-ethyl-2-butanol to the reaction kettle in sequence, stir at 80°C until the sample is completely melted, then heat to 95°C, add 7g of solid heteropoly Acid catalyst, stirred and reacted for 5 hours, opened the dehydration device to fractionate the water generated by the reaction; after the reaction was completed, cooled to room temperature, filtered; the solid heteropolyacid catalyst was recycled, and the filtrate was added to the reactor, and then 1mol 1-ethanolamino- 2-Ethyl-2-butanol neutraliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com