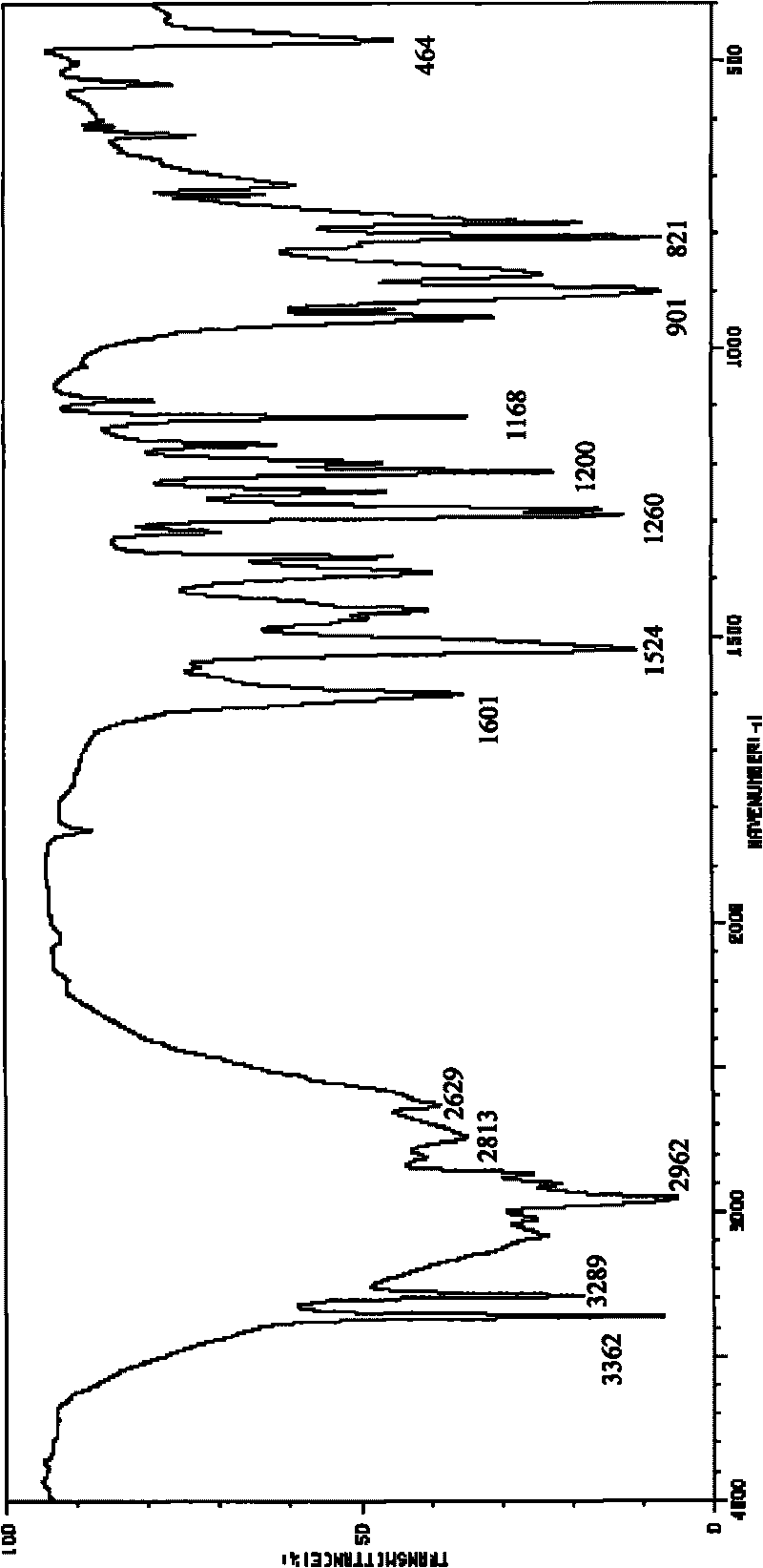
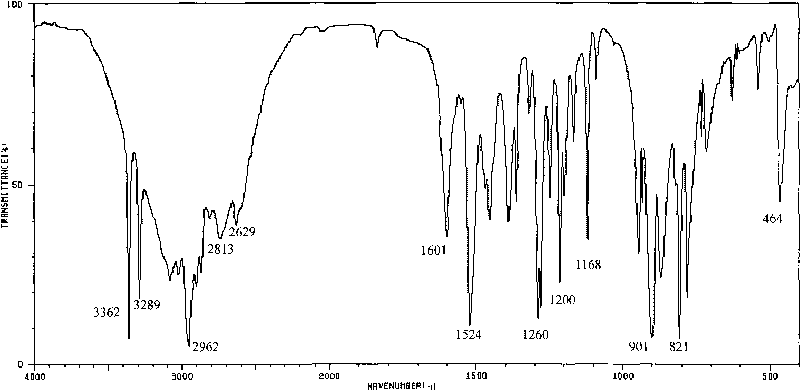
Method for preparing o-amino-p-tertiary butyl phenol
A technology of p-tert-butylphenol and o-amino, which is applied in the field of synthesis of organic compounds, can solve the problems of serious pollution, cumbersome operation, and high cost, and achieve the effects of high reaction yield, easy operation, and small investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, a kind of preparation method of o-amino p-tert-butylphenol, take o-nitro-p-tert-butylphenol as starting raw material, carry out the following steps successively:
[0024] (1) 39.0g (0.2mol) of o-nitro-p-tert-butylphenol, 200mL of 95% ethanol aqueous solution, 2.5g of FeCl 3 ·6H 2 0, 12g gacs are dropped into 500mL there-necked flask together, be warmed up to reflux 15min, then slowly dropwise (20 drops / min) 40g hydrazine hydrate solution (N 2 h 4 ·H 2 The mass percent content of O is 50%, 0.4mol), after the dropwise addition is completed, 2 g of hydrosulfite (sodium dithionite) is added, and the reflux is continued for 3 h.
[0025] (2) After the reaction is completed, filter slightly cold, transfer the filtrate to a 500mL eggplant-shaped flask, add 100mL of water, and distill under reduced pressure to remove ethanol; there is product precipitation, cooling, and filtration; the precipitated product is washed with water and dried to obtain a white flake ...
Embodiment 2
[0027] Embodiment 2, a kind of preparation method of o-amino-p-tert-butylphenol, using o-nitro-p-tert-butylphenol as starting raw material, carries out the following steps successively:
[0028] (1) 39.0g (0.2mol) o-nitro-p-tert-butylphenol, 200mL methanol, 5.0gFeCl 3 ·6H 2 0, 12g gacs are dropped into 500mL there-necked flask together, be warmed up to reflux 15min, then slowly drip 28.6g hydrazine hydrate solution (N 2 h 4 ·H 2 The mass percent content of O is 70%, 0.4 mol), after the dropwise addition, 0.39 g of hydrosulfite (sodium dithionite) is added, and the reflux is continued for 4 hours.
[0029] (2) After the reaction is completed, filter it slightly cold, transfer the filtrate to a 500mL eggplant-shaped flask, add 100mL of water, distill under reduced pressure to remove methanol, and a product precipitates, cools, and filters; the precipitated product is washed with water and dried to obtain a white flake Crystal 32.5g, yield 97.3%, melting point 161-162°C.
[...
Embodiment 3
[0031] Embodiment 3, a preparation method of o-amino-p-tert-butylphenol, using o-nitro-p-tert-butylphenol as a starting material, the following steps are carried out in sequence:
[0032] (1) 39.0g (0.2mol) of o-nitro-p-tert-butylphenol, 200mL of 99% methanol aqueous solution, 1.5g of FeCl 3 ·6H 2 0, 12g gacs are dropped into 500mL there-necked flask together, be warmed up to reflux 15min, then slowly drip 22.2g hydrazine hydrate solution (N 2 h 4 ·H 2 The mass percent content of O is 90%, 0.4 mol), after the dropwise addition is completed, 0.8 g of hydrosulfite (sodium dithionite) is added, and the reflux is continued for 4 hours.
[0033] (2) After the reaction is completed, filter it slightly cold, transfer the filtrate to a 500mL eggplant-shaped flask, add 100mL of water, distill under reduced pressure to remove methanol, and a product precipitates, cools, and filters; the precipitated product is washed with water and dried to obtain a white flake The crystal is 30.9g,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com