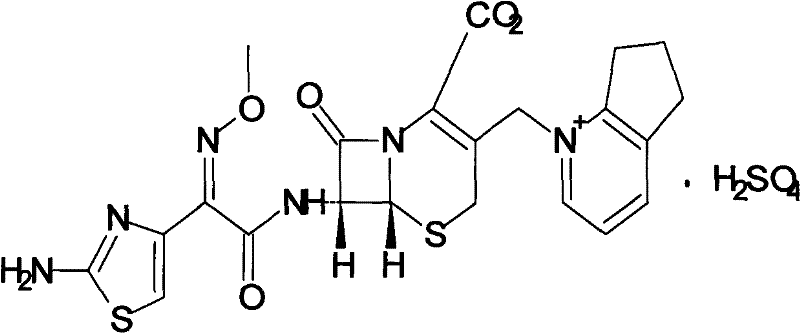
Composition of cefpirome sulfate and sodium citrate
A technology of cefpirome sulfate and sodium citrate, which is applied in the directions of drug delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the structural instability of cefpirome sulfate, side reactions and allergies. High reaction rate, difficult to formulate quality standards and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of powder injection from cefpirome sulfate and sodium citrate composition: in the aseptic ingredient workshop, weigh 50 kg of cefpirome sulfate and sodium citrate aseptic raw material according to the proportioning scheme in Table 1, pour into the high-efficiency three-dimensional In the motion mixer, set the rotation speed of the three-dimensional powder mixer to 5 rpm, start the powder mixing operation according to the powder mixing packaging standard operating procedures, mix for 60 minutes to 90 minutes until the mixture is uniform, discharge, and pack to obtain powder injections.
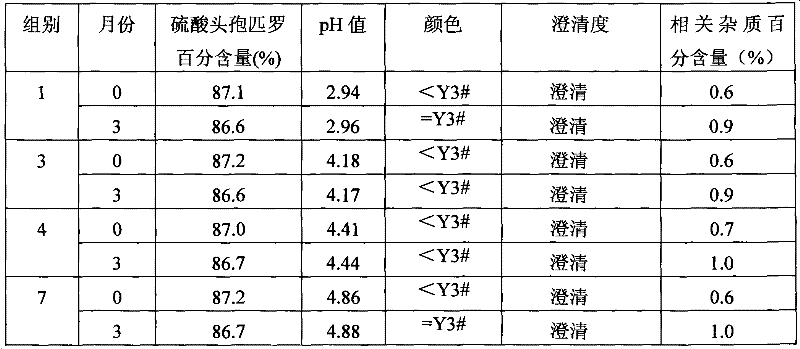
[0020] The samples of each ratio were taken to detect the pH value of each sample, and then the infusion solution with the concentration for routine infusion of adult human body was prepared with water for injection, and the water solubility of each sample solution was tested. The specific experimental results are shown in Table 1.
[0021] The pH value of the cefpirome sulfat...
Embodiment 2
[0024] Embodiment 2 Stability study of cefpirome sulfate and sodium citrate composition
[0025] According to the long-term experiment (3 months) of drug registration standard and the accelerated experiment (3 months) experimental operation standard, detect the stability of cefpirome sulfate and sodium citrate composition that above groups are 1, 3, 4 and 7 , and compared with cefpirome sulfate mixed sodium carbonate. Wherein cefpirome sulfate and the relevant impurity content transformed into by cefpirome sulfate adopt high performance liquid chromatography (HPLC) to detect, use waters2487 type high performance liquid chromatography, use octadecyl bonded silica gel as filler; Acetonitrile-pH3.3 phosphate buffer (weigh 3.45g of ammonium dihydrogen phosphate, add water to dissolve and dilute to 1000ml, adjust pH with phosphoric acid)-water (100:800) as mobile phase; detection wavelength is 270nm. Theoretical plate number should be not less than 1500) with cefpirome peak record...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com