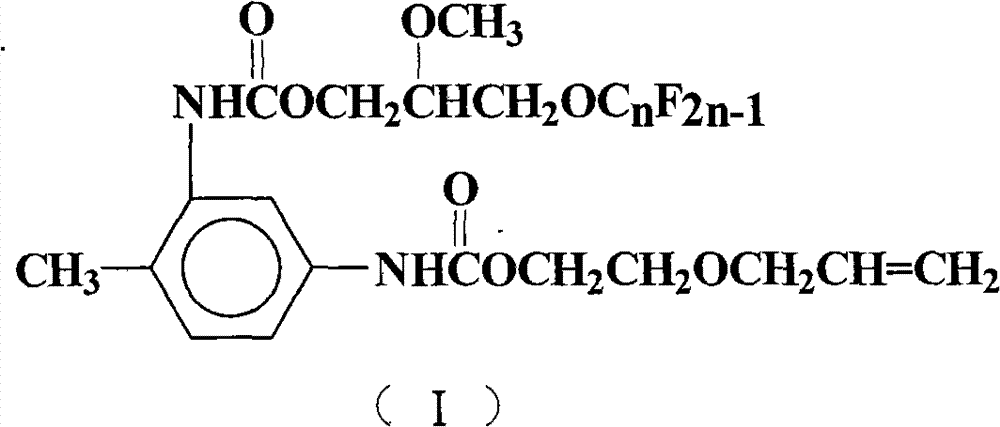
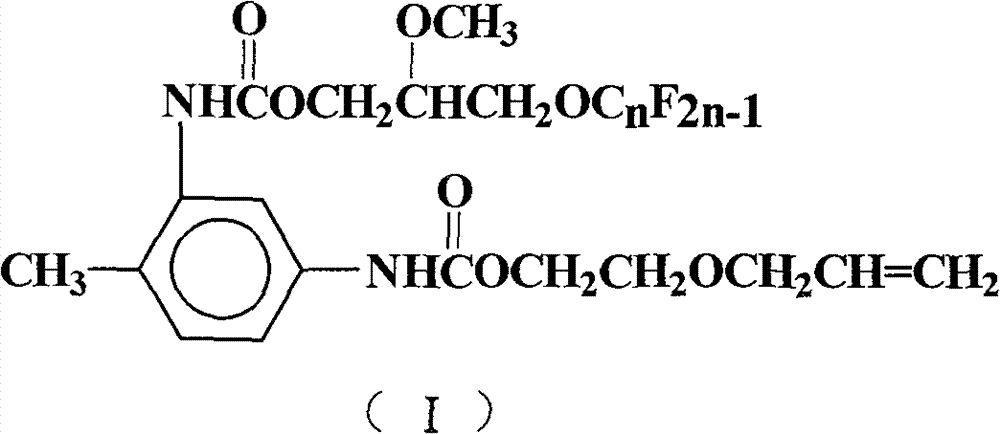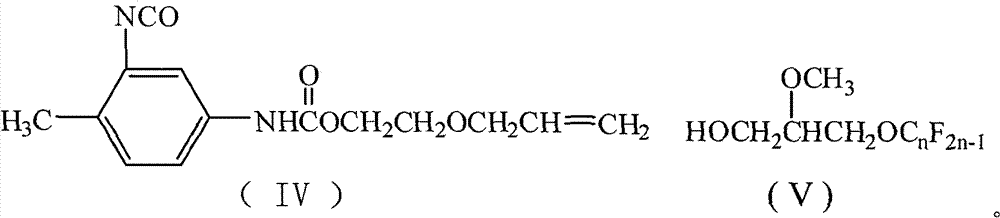Perfluoralkylene-containing allyloxy ethyl carbamate and preparation method and application thereof
A technology of allyloxyethyl carbamate and perfluoroalkenyloxypropanol, which is applied to the preparation of carbamic acid derivatives, the preparation of organic compounds, chemical instruments and methods, etc., to achieve convenient operation, simple synthesis method, The effect of large water contact angle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 50mL of 1,2-dichloroethane, 17.4g (0.1mol) of toluene-2,4-diisocyanate, 1.9g (0.008mol) of tetraethyltin, and 0.5g of hydroquinone into a 250mL four-necked flask. Polymerizing agent, stirred, heated up to 75°C, 10.2g (0.1mol) of ethylene glycol monoallyl ether was added dropwise, after the dropwise addition was completed, TLC was detected, and the reaction process was tracked, and the temperature was kept stirring for 4h. 53.6 g (0.1 mol) of 2-methoxy-3-perfluorononenyloxypropanol was added dropwise. After the dropwise addition was completed, TLC detection was carried out to track the reaction progress, and the reaction was carried out while maintaining the temperature and stirring for 5 h. Reaction is completed, and solvent extraction is separated by silica gel column chromatography (V 环己烷 :V 乙酸乙酯 =7:3), after removing the solvent, yellow viscous paste I was obtained 1 (n=9), the perfluoroalkenyl-containing acrylate represented by formula (I), the yield is 66.8%,...
Embodiment 2
[0033] React according to the method of Example 1, but 2-methoxyl-3-perfluorononenyloxypropanol is changed to 2-methoxyl-3-perfluorohexenyloxypropanol 38.6g (0.1mol), Other reaction conditions are identical with embodiment 1, obtain pale yellow paste I 2 (n=6), yield 61.0%, purity 99.3%.
[0034] 19 F NMRδ(CDCl 3 ): -129.4(t, 3F), -127.4(s, 3F), -121.6(m, 2F), -83.3(s, 2F), -78.1(t, 1F).
Embodiment 3
[0036] Add 50mL of N,N-dimethylacetamide, 17.4g (0.1mol) of 2,4-TDI, 4.27g (0.01mol) of tetraphenyltin into a 250mL four-necked flask, and 0.8 p-hydroxyanisole as a polymerization inhibitor , stirred, the temperature was raised to 100°C, and 15.3 g (0.15 mol) of ethylene glycol monoallyl ether was added dropwise. After the completion of the dropwise addition, TLC was detected to track the reaction process, and the temperature was kept to stir for 4 hours. 80.4 g (0.15 mol) of 2-methoxy-3-perfluorononenyloxypropanol was added dropwise. After the dropwise addition was completed, TLC detection was carried out to track the reaction progress, and the reaction was carried out while maintaining the temperature and stirring for 5 h. After completion of the reaction, desolvation, silica gel column chromatography
[0037] (V 环己烷 :V 乙酸乙酯 = 7: 3), the yellow paste I was obtained after removing the solvent 1 (n=9), yield 69.2%, purity 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com