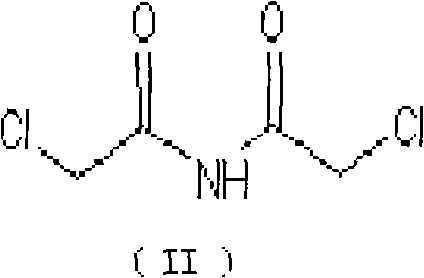Preparation method of dexrazoxane and pharmaceutical salts thereof
A technology for delazox and medicinal salts, which is applied in the field of preparation of delazox or its pharmaceutically acceptable salts, can solve the problems of low purity, low yield, patient side effects, etc., and achieves reasonable process design and product purity. High and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0047] Add 1193g of chloroacetonitrile into a 3000ml three-necked reaction flask, add 3580g of saturated hydrogen chloride methanol solution, stir at 25°C for 8 hours, heat up to 125°C for 1 hour, cool to 30°C, pour into 3000ml of water, stir for 2 hours, filter, Purified with ethanol to obtain 843.7g of N,N-dichloroacetimide (II), mp=195-197°C.
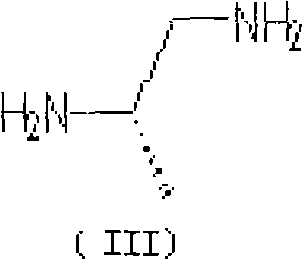
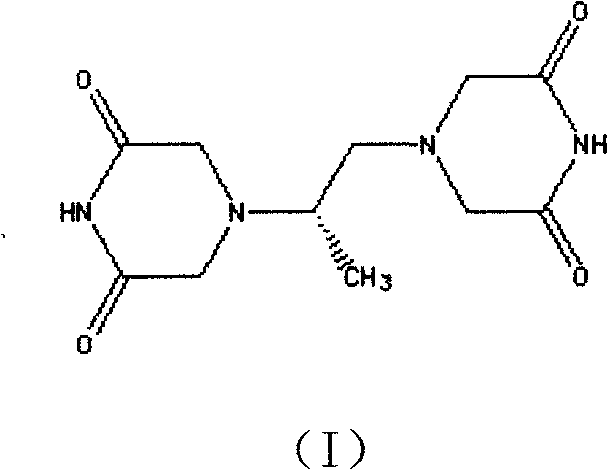
[0048] In a 2000ml three-necked reaction flask, add 170g of the above-mentioned N,N-dichloroacetimide (II) and 2040g of methanol, add 40g of sodium ethoxide under stirring, mix and dissolve for 1 hour, and dropwise add 34g of dextrorotatory 1,2-propylenediamine And 510g of ethanol solution, heated to 50°C for 4 hours, cooled to 45°C, added 35g of sodium ethylate, heated to 80°C to continue the reaction for 4 hours, during the reaction, crystals slowly precipitated, cooled to 20 ℃, filter, wash with ethanol, filter dry, and vacuum-dry at 60-80 ℃ to obtain 160.4 g of Delazolate (I). (mp=192°-195°; Optical Rotation: [a]D+11.35°-+11.40°...
example 2
[0051] Add 1193g of chloroacetonitrile into a 3000ml three-necked reaction flask, add 3580g of sulfuric acid, stir at 25°C for 8 hours, heat up to 100°C for 1 hour, cool to 30°C, pour into 3000ml of water, stir for 2 hours, filter, and refine with ethanol. 840.3 g of N,N-dichloroacetimide (II) were obtained, mp=194-196°C.
[0052] In a 2000ml three-necked reaction flask, add 170g of the above-mentioned N,N-dichloroacetimide (II) and 510g of methanol, add 40g of potassium carbonate under stirring, mix and dissolve for 1 hour, and add 17g of dextrorotatory 1,2-propanediamine dropwise And 3400g of ethanol solution, heated up to 30°C to react for 4 hours, added 35g of sodium ethylate, heated to 80°C to continue reaction for 4 hours, during the reaction, crystals slowly precipitated, cooled to 20°C, filtered, ethanol Wash, filter dry, and vacuum-dry at 60-80° C. to obtain 156.8 g of Delazolate (I). (mp=192°-196°; Optical Rotation: [a]D+11.30°-+11.40° (c=5, DMF); total yield 74.3%)...
example 3
[0055] Add 1193g of chloroacetonitrile and 597g of phosphoric acid into a 3000ml three-necked reaction flask, stir at 25°C for 8 hours, heat up to 150°C for 1 hour, cool to 30°C, pour into 3000ml of water, stir for 2 hours, filter, and refine with ethanol. 835.5 g of N,N-dichloroacetimide (II) were obtained, mp=195-198°C.
[0056] In a 2000ml three-necked reaction flask, add 170g of the above-mentioned N,N-dichloroacetimide (II) and a mixture of 3400g of methanol and propanol (mass ratio 1:1), add 40g of sodium carbonate under stirring, stir, mix and dissolve for 1 hour , add dropwise a solution of 51g of dextrorotatory 1,2-propylenediamine and 2040g of ethanol, heat up to 80°C for 4 hours, cool to 45°C, add 35g of sodium ethylate, heat up to 80°C to continue the reaction for 4 hours after adding, During the reaction process, crystals were slowly precipitated, cooled to 20°C, filtered, washed with ethanol, filtered and dried, and vacuum-dried at 60-80°C to obtain 152.3g of Del...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com