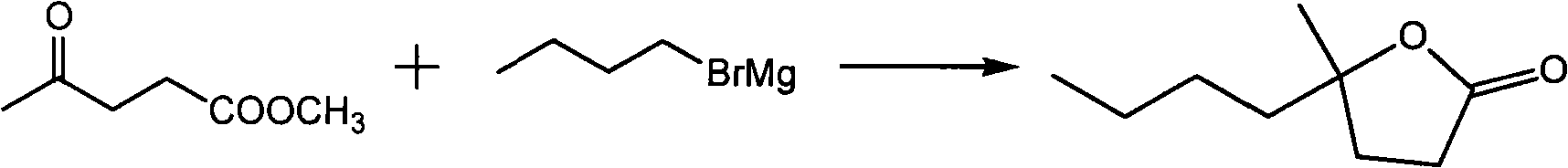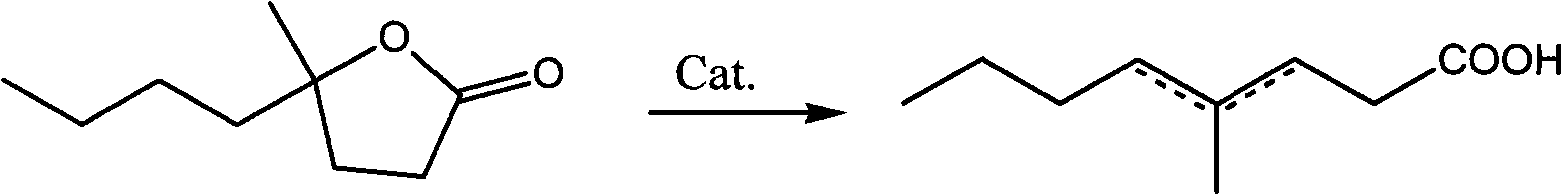4-Method for synthesizing 4-Methyloctanoic Acid
A synthesis method and technology of methyl octanoic acid, applied in the preparation of carboxylate/lactone, organic chemistry, etc., can solve the problems of high difficulty, rare raw materials, and high preparation cost, and achieve reduced difficulty, mild reaction conditions, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
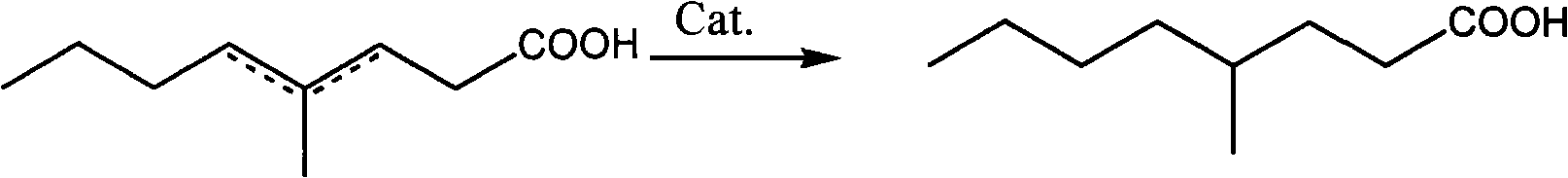
[0015] Embodiment 1: the synthesis of 4-methyl-butylactone
[0016] In a 2L flask equipped with a mechanical stirrer, a thermometer, an addition funnel and a reflux condenser, add 48g (2mol) of magnesium shavings, 150mL of diethyl ether and a grain of iodine. Start stirring, add dropwise a mixture of 274g bromobutane (2mol) and 300mL diethyl ether, keep the reaction slightly boiling, complete the addition in about 2 hours, and then keep the temperature at 40°C for 1 hour. Cool the reaction liquid to -5°C, add dropwise a mixture of 260g methyl levulinate (2mol) and 150mL diethyl ether, and control the reaction temperature below 0°C. After the addition, stir at room temperature for 2 hours, carefully add 416g of saturated ammonium chloride aqueous solution to decompose, control the temperature at 10°C, and generate granular solid and almost dry ether layer. Pour off the ether layer, extract the solid with 2*250mL ether, distill the crude product under reduced pressure after dee...
Embodiment 2
[0017] Embodiment 2: the synthesis of 4-methyl-butylactone
[0018] In a 1L flask equipped with a mechanical stirrer, a thermometer, an addition funnel and a reflux condenser, add 24 g (1 mol) of magnesium shavings, 75 mL of diethyl ether and a grain of iodine. Start stirring, add dropwise a mixture of 140 g of bromobutane (1 mol) and 150 mL of diethyl ether, keep the reaction slightly boiling, complete the addition in about 2 hours, and then keep the temperature at 40°C for 1 hour. Cool the reaction liquid to -15°C, add dropwise a mixture of 156g methyl levulinate (1.2mol) and 150mL diethyl ether, and control the reaction temperature below -10°C. After the addition, stir at room temperature for 2 hours, carefully add 208 g of saturated ammonium chloride aqueous solution to decompose, control the temperature at 10 ° C, and generate granular solid and almost dry ether layer. Pour off the ether layer, extract the solid with 2*125mL ether, distill the crude product under reduced...
Embodiment 3
[0019] Embodiment 3: the synthesis of 4-methyl-butylactone
[0020] In a 2L flask equipped with a mechanical stirrer, a thermometer, an addition funnel and a reflux condenser, add 24 g (1 mol) of magnesium alloy, 100 mL of tetrahydrofuran, benzene (tetrahydrofuran:benzene=1:1) and a grain of iodine. Start stirring, add dropwise a mixture of 140g of bromobutane (1mol) and 200mL of tetrahydrofuran and benzene, keep the reaction temperature at about 45°C, complete the addition in about 2 hours, and then keep the temperature at 45°C for 1 hour. Cool the reaction liquid to -10°C, add dropwise a mixture of 156g of methyl levulinate (1.2mol) and 150mL of tetrahydrofuran and benzene, and control the reaction temperature below -5°C. After the addition, stir at room temperature for 2 hours, carefully add 350g of 10% hydrochloric acid to decompose, control the temperature at 10°C, wash the base layer with water until neutral, remove the solvent and distill the crude product under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com