Polyaphron topical composition with vitamin d and corticosteroid
A technology of corticosteroids and compositions, applied in the field of topical compositions, which can solve the problems of poor diffusion, unacceptable to patients, unfavorable, etc., and achieve the effect of happy application, enhanced skin permeability, and reduced possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
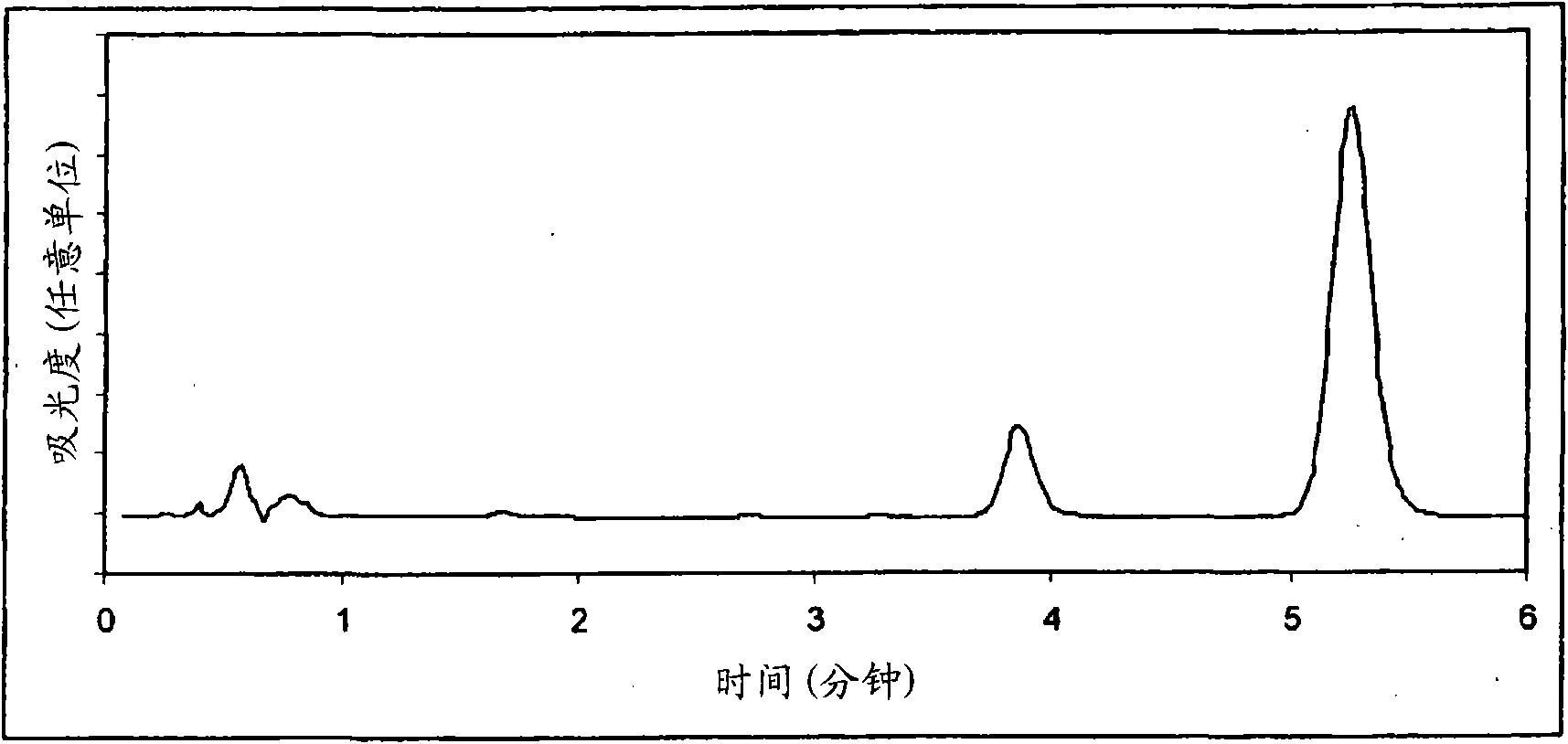
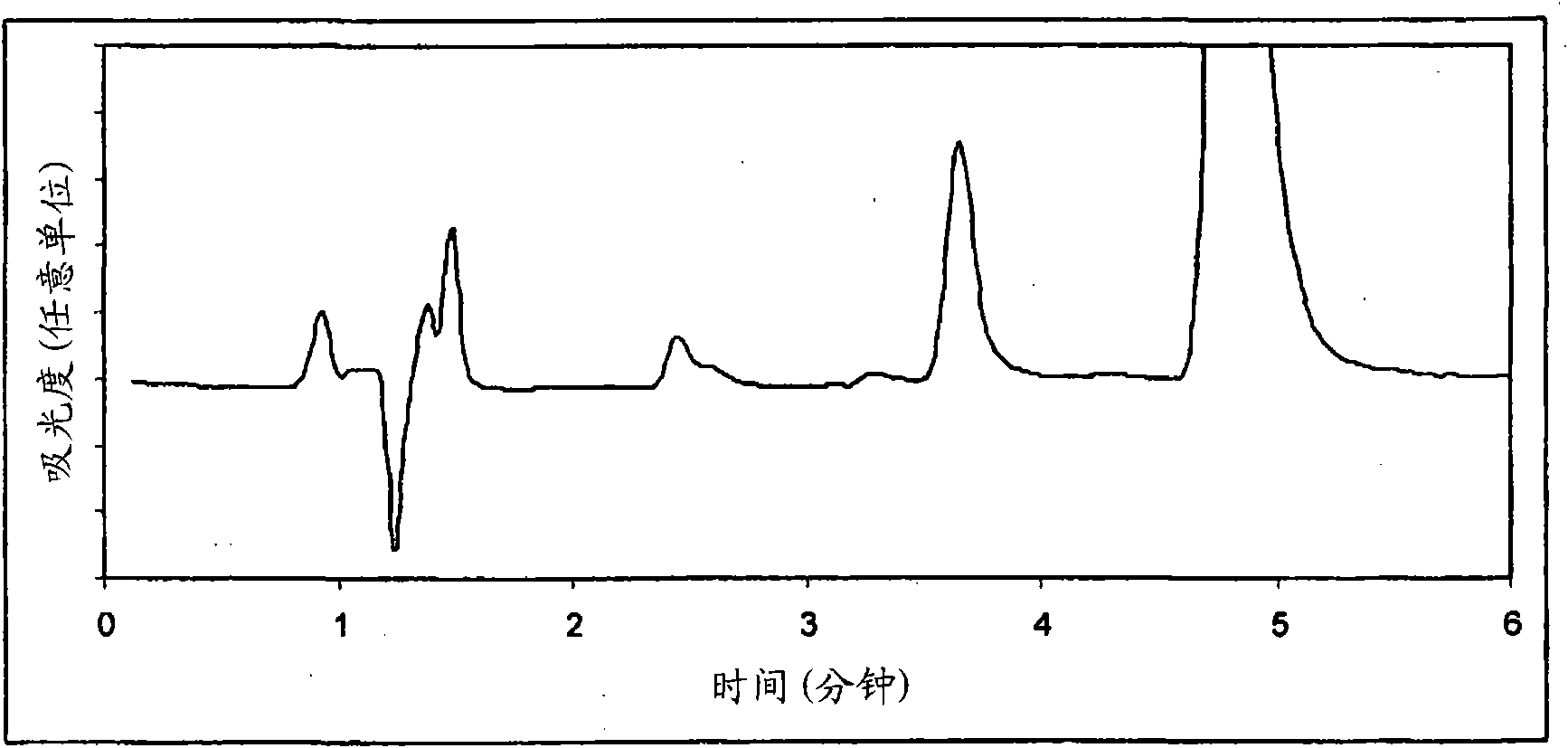
Image
Examples
Embodiment 1
[0121] Three gel polyaphron dispersions with the compositions described below were prepared by the following methods.
[0122] Gel polymicrobubble dispersion 1
%
Calcipotriol solution in caprylic / capric triglyceride (Mygliol 812-Condea) *
(0.0695%)
89.10
Laureth-4 (Volpo L4-Croda)
0.90
water box
Poloxamer 188 (Pluronic F68-BASF)
1.00
9.00
[0123] Gel polymicrobubble dispersion 2
%
Betamethasone dipropionate in caprylic / capric triglyceride (Mygliol 812-Condea)
the solution ** (0.3465%)
89.10
Laureth-4 (Volpo L4-Croda)
0.90
water box
Poloxamer 188 (Pluronic F68-BASF)
1.00
9.00
[0124] Gel polymicrobubble dispersion 3
weight%
Squalane (from Olive - A&E Connock)
9.00 ...
Embodiment 2
10.05
water gel
9.88
[0161] * The final calcipotriol level was 55.4 μg / g.
[0162] ** The final BDP level was 645 μg / g, equivalent to 502 μg / g.
[0163] Manufacturing method
[0164] The method used was as described above in Example 1, except that propylene glycol and the neutralized aqueous gel were added to the final mixture of the three gelling polyaphron dispersions 1, 2 and 3, which were then Proceed to Mix: Mix briefly until the product is a homogeneous mixture of microcellular dispersions.
[0165] Neutralized gels were obtained by adding triethanolamine (a base) to a dispersion of polyacrylic acid to form a clear gel with a pH of 7.5 ± 0.2. Methods for neutralizing polyacrylic acid gels are well known to those skilled in the art.
[0166] Stability measurement
[0167] Stability was tested as in Example 1b.
[0168] The inventors observed that the levels of calcipotriol and betamethasone dipropionate were 104% ± ...
Embodiment 3
[0172] Three gel polyaphron dispersions and aqueous gels of the compositions described below were prepared by the following methods.
[0173] Gel polymicrobubble dispersion 1
oil phase
%
Isopropyl myristate (Lexol IPM NF-Inolex) in a weight ratio of 3:2: caprylic / capric
Calcipotriol solution in a blend of triglycerides (Mygliol 812-Condea) * (0.0573%)
89.10
Laureth-4 (Volpo L4-Croda)
0.90
water box
Poloxamer 188 (Pluronic F68-BASF)
1.00
9.00
[0174] Gel polymicrobubble dispersion 2
oil phase
%
Betamethasone dipropionate dissolved in caprylic / capric triglyceride (Mygliol 812-Condea)
liquid ** (0.3838%)
89.10
Laureth-4 (Volpo L4-Croda)
0.90
water box
Poloxamer 188 (Pluronic F68-BASF)
1.00
softened water
9.00
[0175] Gel polymicrobub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com