Ferrous fumarate folic acid dispersible tablet and preparation method thereof
A technology of ferrous fumarate and dispersible tablets, which is applied to medical preparations, pharmaceutical formulations, organic active ingredients and other directions containing active ingredients, can solve problems such as no research reports on compound dispersible tablets, and achieve the prevention of iron deficiency anemia. , the production process is simple and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The ferrous fumarate folic acid dispersible tablet of preferred embodiment, its preparation method comprises the following steps:
[0040] a, pulverization: respectively pulverize ferrous fumarate and folic acid into fine powder with a fineness of more than 120 mesh, and pulverize the pharmaceutically acceptable carrier into fine powder with a fineness of more than 100 mesh;
[0041] b. Granulation: Mix ferrous fumarate, folic acid, filler and internal disintegrating agent after step a is pulverized (surfactant can also be added), then add binder to make soft material, 18~ Granulate with 24 mesh sieve, dry at 45℃~55℃, pass through 20~24 mesh sieve for granulation;
[0042] c, tabletting: add lubricant and additional disintegrating agent (can also add flavoring agent) to the dry granule after step b granulation, mix evenly, tabletting, obtains ferrous fumarate folic acid dispersible tablet.
[0043] Because the dispersible tablet of the present invention has higher drug...
Embodiment 1
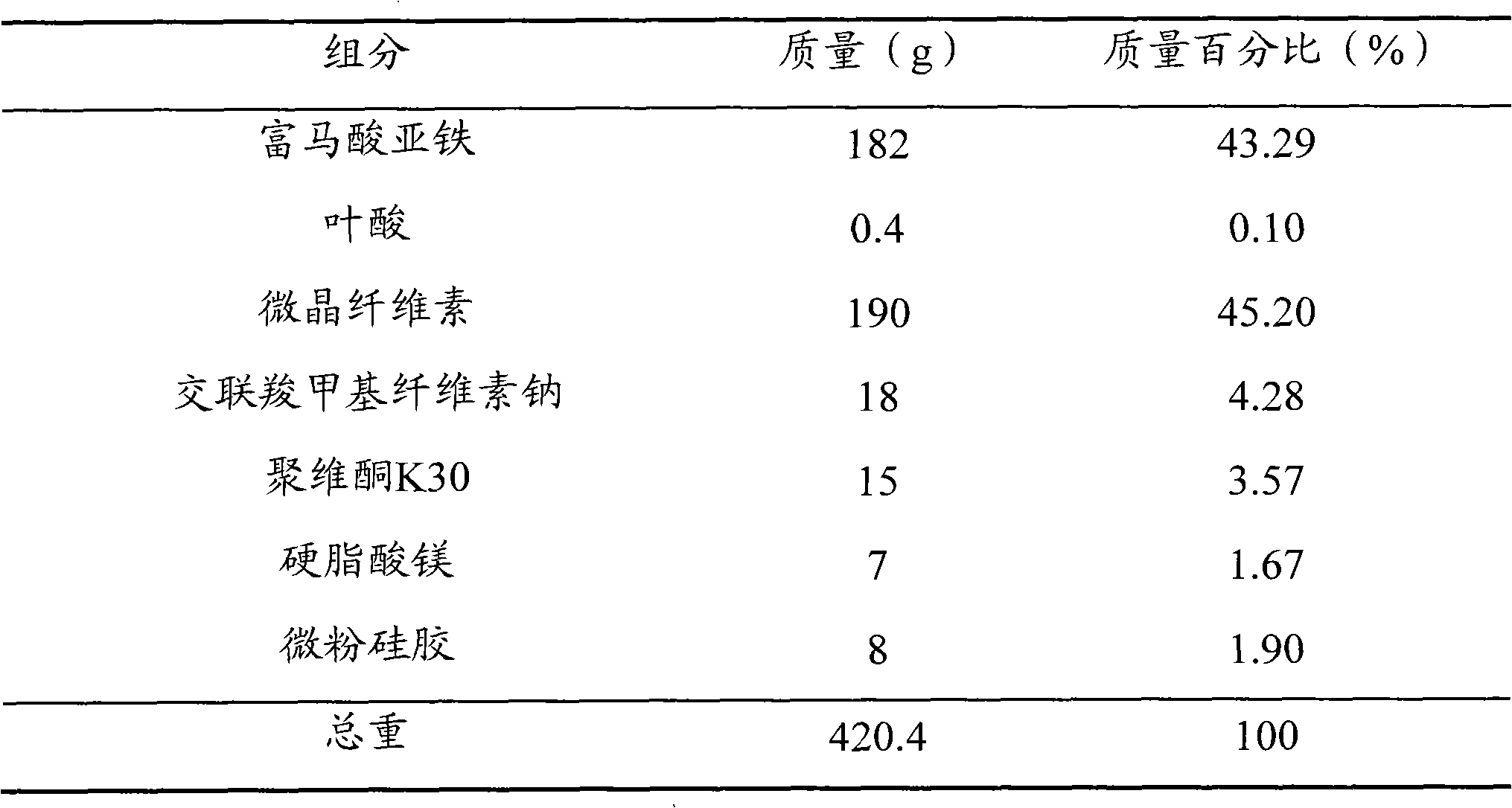
[0046] prescription
[0047]
[0048] method Ferrous fumarate and folic acid were pulverized into fine powders with a fineness above 120 mesh respectively, and the remaining components were respectively pulverized into fine powders with a fineness above 100 mesh; 182 g of ferrous fumarate, 0.4 g of folic acid, microcrystalline Mix 190g of cellulose and 18g of croscarmellose sodium, add an aqueous solution of povidone K30 with a mass fraction of 2% to make a soft material, granulate with a 24-mesh sieve, dry at 50°C, and pass the dry granules through a 24-mesh sieve Whole granules; add 7g of magnesium stearate and 8g of micropowdered silica gel to the dry granules, mix well, and press into tablets to make 1000 ferrous fumarate folic acid dispersible tablets, each containing 60mg of iron and 0.4mg of folic acid.
[0049] quality The obtained dispersible tablet is smooth and shiny in appearance; it disintegrates completely within 2.1 minutes in water at 20 ± 1°C, is u...
Embodiment 2
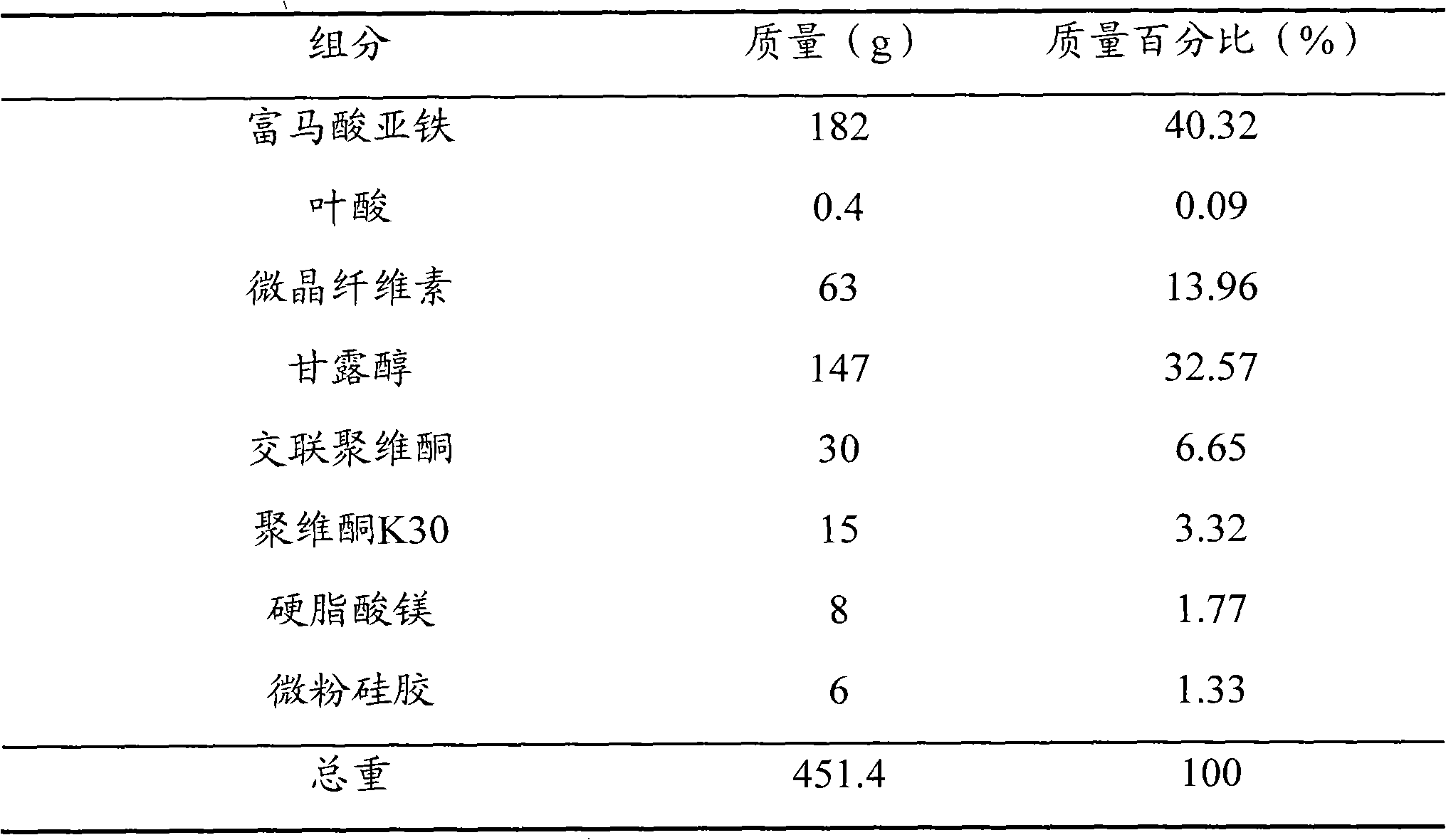
[0051] prescription
[0052]
[0053] method Ferrous fumarate and folic acid were pulverized into fine powders with a fineness above 120 mesh respectively, and the remaining components were respectively pulverized into fine powders with a fineness above 100 mesh; 182 g of ferrous fumarate, 0.4 g of folic acid, microcrystalline Mix 63g of cellulose, 147g of mannitol, and 30g of crospovidone, add an aqueous solution of povidone K30 with a mass fraction of 2% to make a soft material, granulate with a 24-mesh sieve, dry at 50°C, and pass through a 24-mesh dry granule Sieve the granules; add 8g of magnesium stearate and 6g of micropowdered silica gel to the dry granules, mix well, and press into tablets to make 1,000 ferrous fumarate and folic acid dispersible tablets, each containing 60mg of iron and 0.4mg of folic acid.
[0054] quality The obtained dispersible tablets are smooth and shiny in appearance; they disintegrate completely in 1.5 minutes in water at 20 ± 1°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fineness | aaaaa | aaaaa |
| Fineness | aaaaa | aaaaa |
| Fineness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com