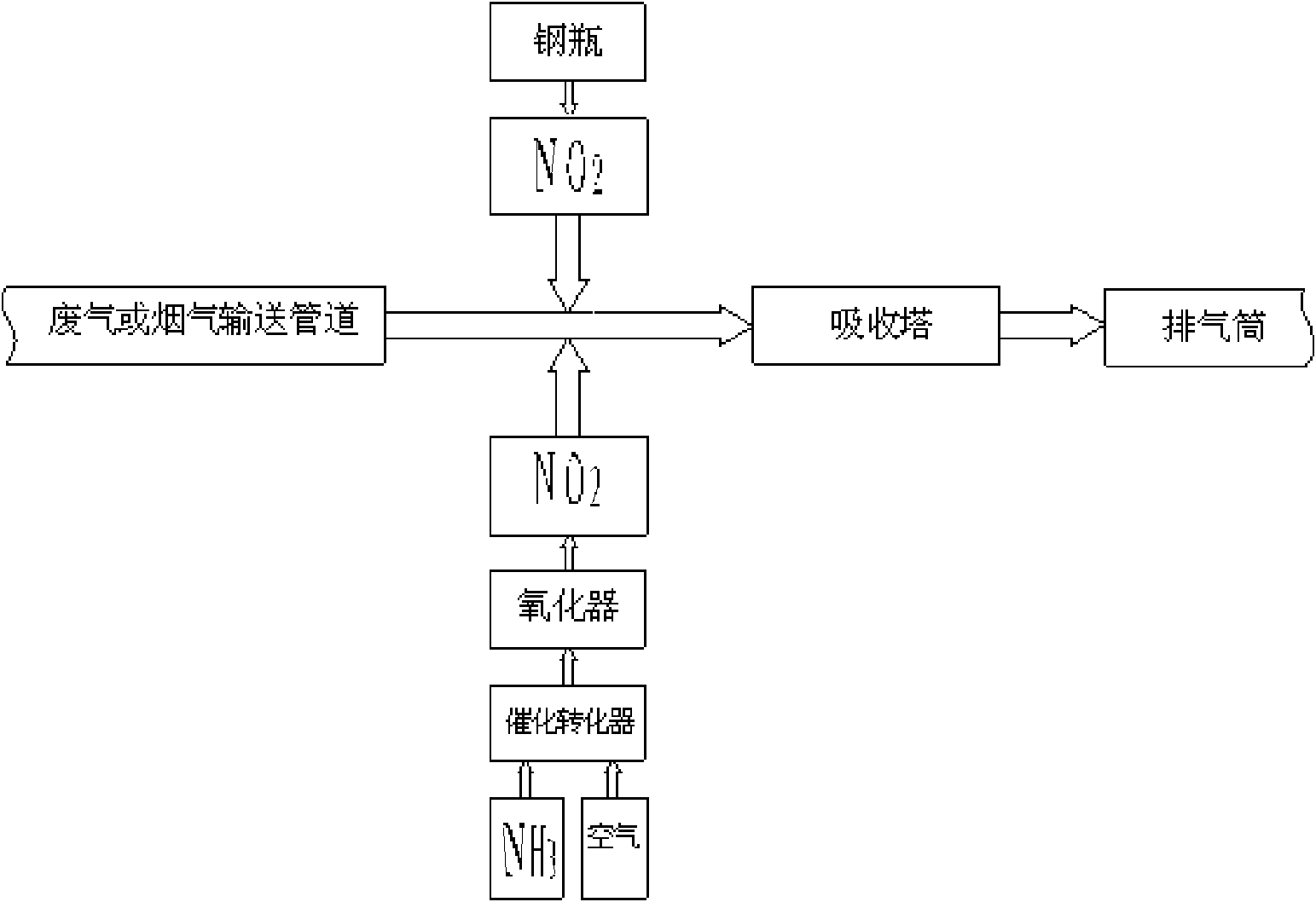
Method for removing nitrogen oxides in waste gas or flue gas
A nitrogen oxides and waste gas technology, applied in chemical instruments and methods, separation methods, dispersed particle separation, etc., can solve the problems that have not yet seen ideal reports, the direct oxidation rate of NO is slow, and the conversion rate per unit time is low. Abundant raw materials, low removal cost, easy to popularize and popularize in a large area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
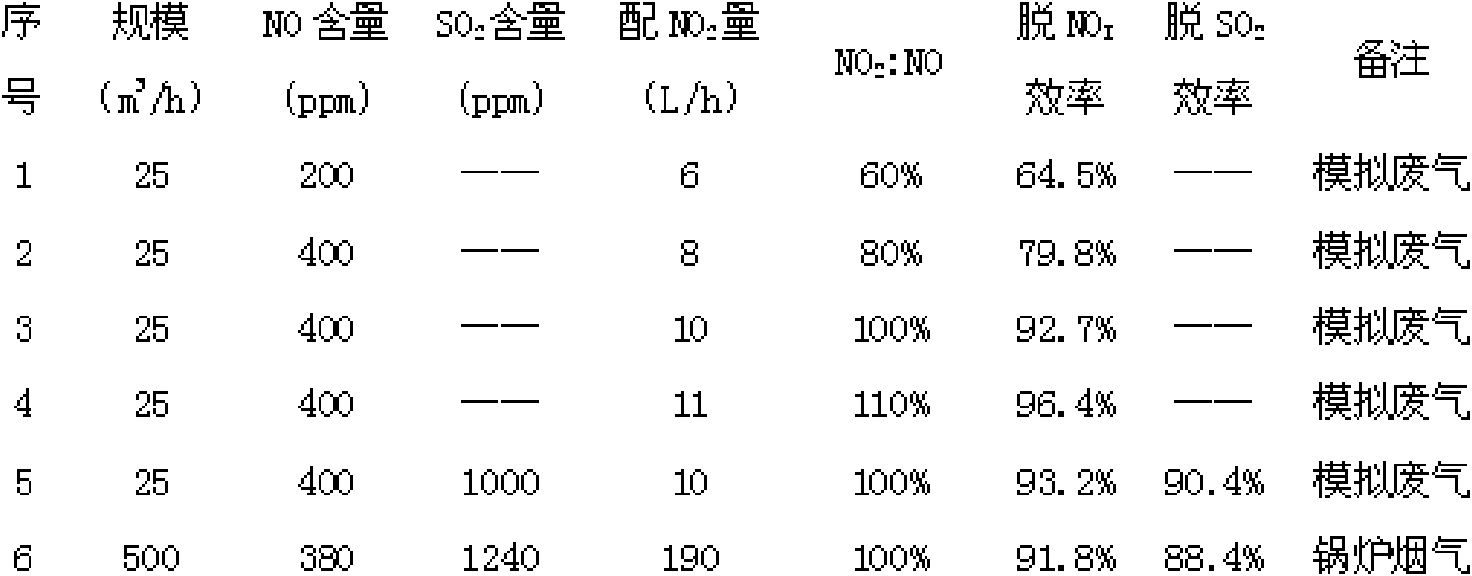
[0038] 25 cubic meters per hour (m 3 / h) simulated exhaust gas, the simulated exhaust gas contains 200ppm NO, and the rest is air, with 6 liters per hour (L / h) NO 2 gas, NO 2 The volume ratio to NO is 60%, and the lime slurry with a mass fraction of 10% is used as the absorbent, the circulation rate is 25L / h, and the NO x The removal efficiency is 64.5%.
Embodiment 2
[0040] In an absorption tower with a diameter of 100mm, a built-in 2-layer sieve plate, an opening rate of 17%, and a small hole diameter of 2mm, the 25m 3 / h simulated exhaust gas, the simulated exhaust gas contains 400ppm of NO, the rest is air, with 8L / h NO 2 gas, NO 2 The volume ratio to NO is 80%, and the lime slurry with a mass fraction of 10% is used as the absorbent, the circulation rate is 25L / h, and the NO x The removal efficiency is 79.8%.
Embodiment 3
[0042] In an absorption tower with a diameter of 100mm, a built-in 2-layer sieve plate, an opening rate of 17%, and a small hole diameter of 2mm, the 25m 3 / h simulated exhaust gas, 400ppm NO in the simulated exhaust gas, the rest is air, with 10L / h NO 2 gas, NO 2 The volume ratio to NO is 100%, and the lime slurry with a mass fraction of 10% is used as the absorbent, the circulation rate is 25L / h, and the NO x The removal efficiency is 92.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com