Method for determining amount of residual florfenicol in aquatic product by using high-efficiency liquid chromatogram fluorescence method
A technology of high performance liquid chromatography and florfenicol, which is applied in the field of detection of florfenicol residues in aquatic products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Extraction
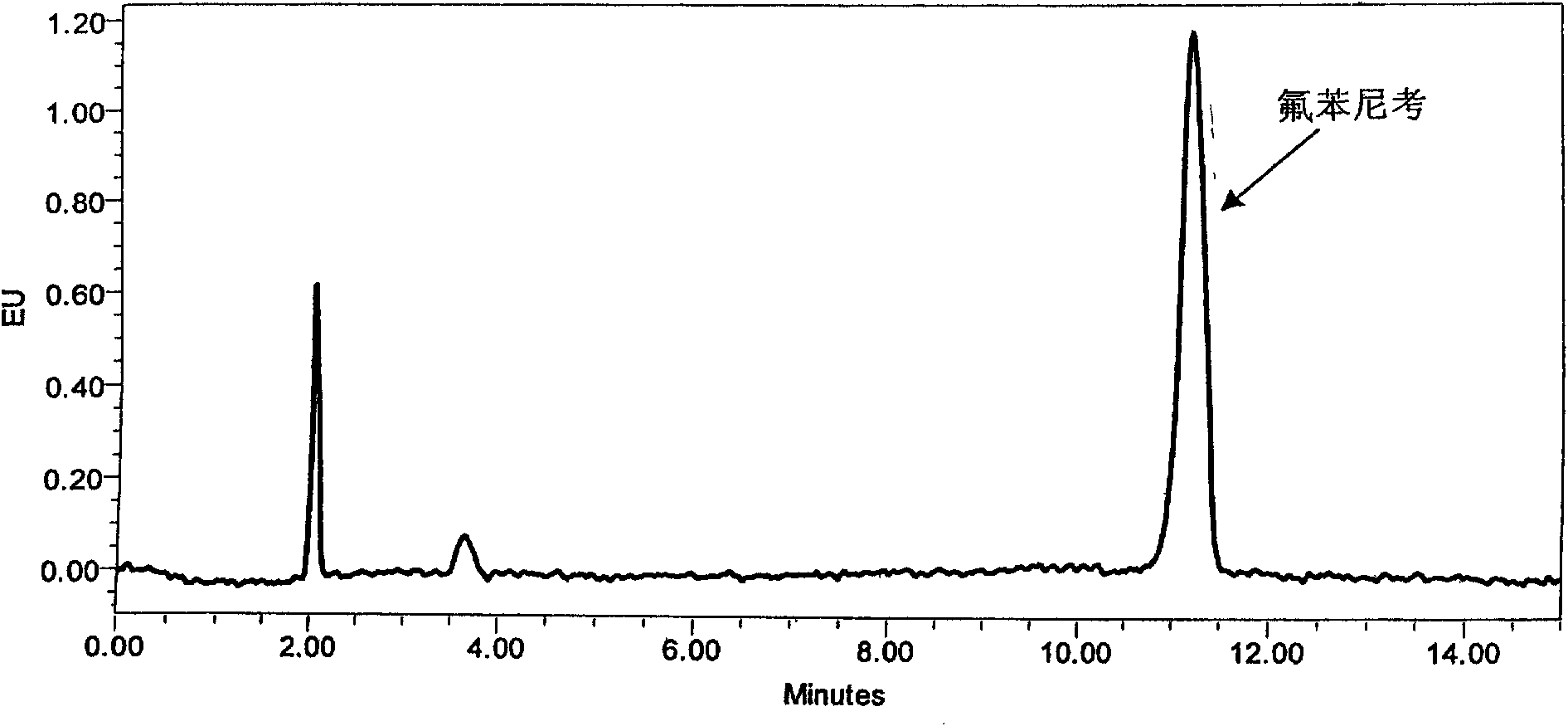
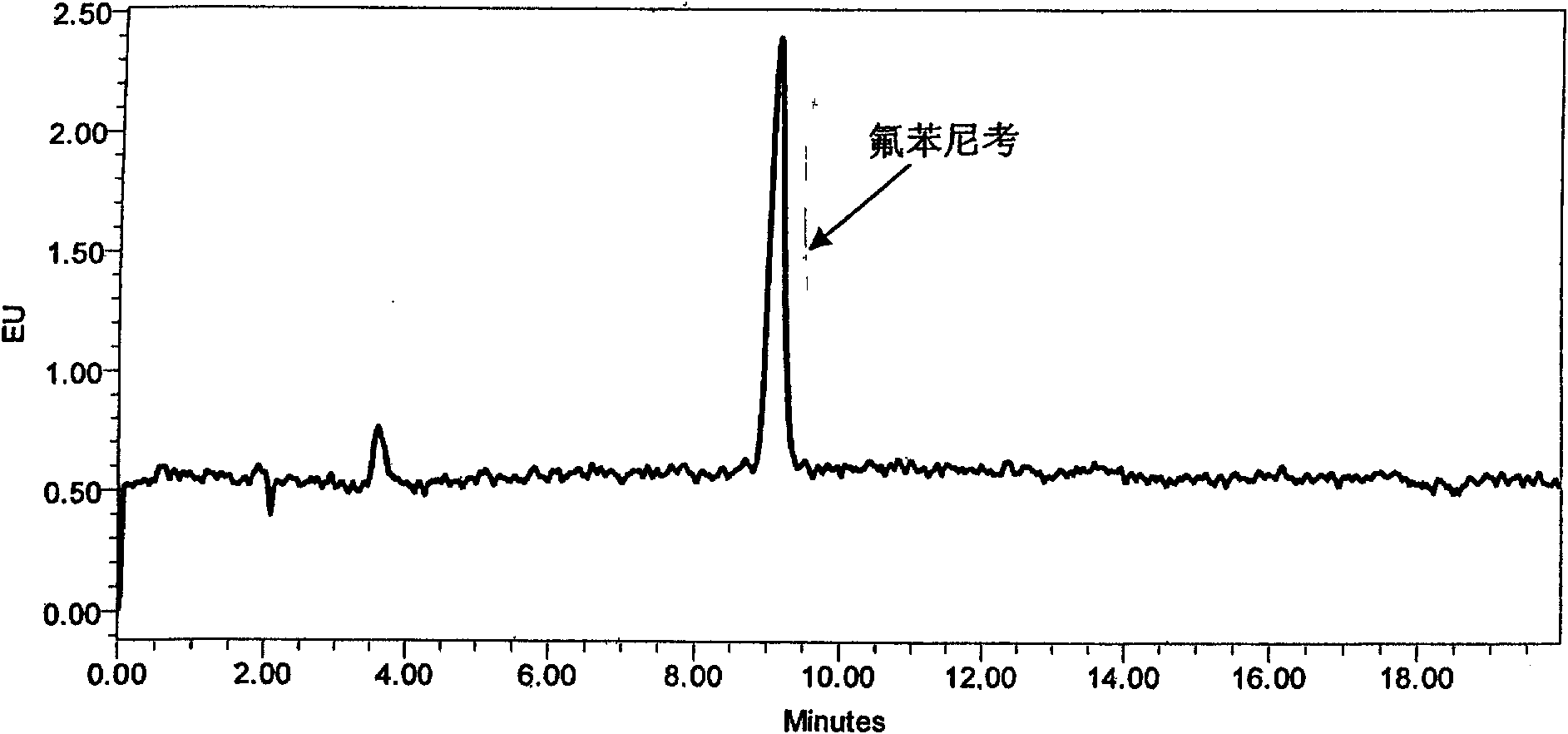
[0048] Since the different ratios of acetonitrile and water will affect the retention time of the chromatographic peaks, in order to ensure that the peak time of Florfenicol is ideal and the peak shape is sharp, the ratio of acetonitrile and water in the mobile phase is finally determined as 25:75. The peak eluting time is ideal, and the target peak and impurity peak can be completely separated. As increasing the proportion of mobile phase acetonitrile, although the analysis time can be shortened, the peak time of florfenicol is too early, and it is difficult to separate completely from the impurity peak ( figure 2 ). As reducing the proportion of acetonitrile, the peak time of florfenicol is later, and the analysis time is longer ( image 3 ).
[0049] Therefore, in order to ensure that each target has a good separation effect and chromatographic peak shape, this method uses acetonitrile+water (25:75) solution as the mobile phase, and adopts...
Embodiment 2
[0050] Example 2: Purification
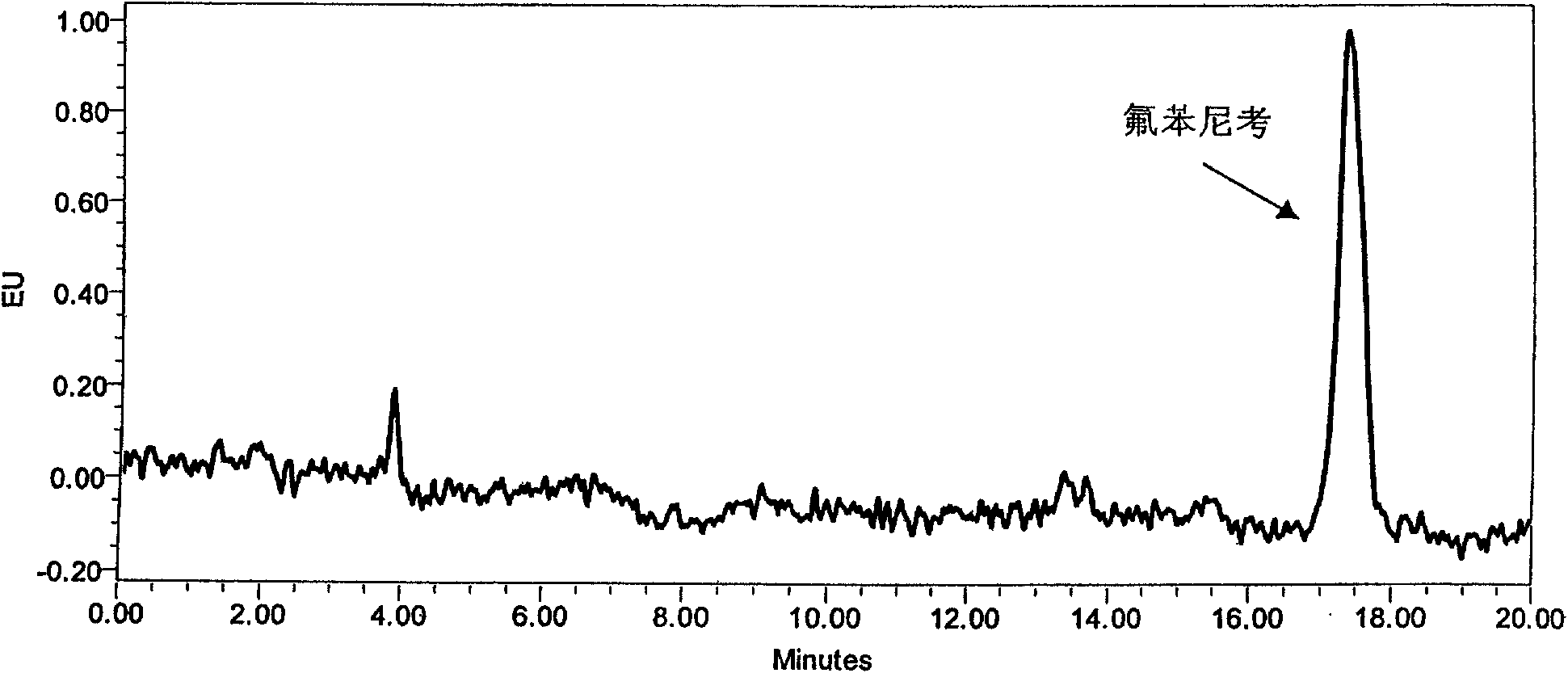
[0051] Many documents are processed to degreasing and purification, then concentrate, constant volume, and test on the machine. This study has also done a lot of experiments in this area, and found that the presence of some substrates interfered with the qualitative and quantitative peaks of the target drug and reduced the detection sensitivity when detecting low-concentration drugs ( Figure 4 ). Therefore, it is not enough to degrease and purify only by n-hexane, because n-hexane only removes most of the non-polar impurity components, and many polar substances, such as phospholipids, glycoproteins, etc., still exist in the extraction solvent, so further SPE purification is required . On the basis of comparing different types, solid-phase extraction columns of different types of fillers and different filler amounts on the purification effect of the target compound, it is concluded that the purification effect of the C18 solid-phase extractio...
Embodiment 3
[0052] Example 3: Method linear range and correlation
[0053] Take a certain amount of 1.0mg / L standard intermediate solution, dilute it with acetonitrile in sequence to 0.2, 0.5, 5, 10, 50, 100mg / L standard working solution, and detect according to the above chromatographic conditions. Carry out linear regression analysis to the mass concentration X (mg / L) of standard working solution with the peak area Y of each concentration, linear regression equation Y=225043X+82815, correlation coefficient r=0.9996 ( Figure 6 ). The results showed that: Florfenicol had a good linear relationship in the concentration range of 0.2μg / mL~100μg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com